424B5: Prospectus filed pursuant to Rule 424(b)(5)
Published on March 8, 2018
|
|
Filed Pursuant to Rule 424(b)(5) |
||||||
|
Registration No. 333-206781 | |||||||
|
SUBJECT TO COMPLETION, DATED MARCH 8, 2018 | |||||||
|
PROSPECTUS SUPPLEMENT | |||||||
|
(To Prospectus dated September 18, 2015) | |||||||
|
| |||||||
|
| |||||||
|
| |||||||
|
VOLITIONRX LIMITED | |||||||
|
| |||||||
|
Shares of Common Stock | |||||||
|
| |||||||
|
We are offering shares of our common stock. Our common stock is listed on the NYSE American under the symbol “VNRX.” On March 7, 2018, the last reported sale price of our common stock was $2.86 per share. | |||||||
|
| |||||||
|
Investing in our common stock involves a high degree of risk. You should carefully review the risks and uncertainties described under the heading “Risk Factors” beginning on page S-5 of this prospectus supplement. | |||||||
|
| |||||||
|
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus supplement or the accompanying prospectus is truthful or complete. Any representation to the contrary is a criminal offense. | |||||||
|
______________________________ | |||||||
|
|
|
Per Share |
|
|
Total |
|
|
|
Public offering price |
$ |
|
|
$ |
|
|
|
|
Underwriting discount(1) |
$ |
|
|
$ |
|
|
|
|
Proceeds, before expenses, to us |
$ |
|
|
$ |
|
|
|
|
(1) Gives effect to the fact that the underwriting discount for shares sold to (A) officers, directors and certain specified existing investors of VolitionRx, or the insiders, shall be 3% of the aggregate purchase price, and (B) all other investors in this offering shall be 6% of the aggregate purchase price; subject to a minimum blended discount of 5% of the aggregate purchase price so long as at least 50% of the aggregate purchase price is from investors other than insiders. See “Underwriting” for additional disclosure regarding underwriting discounts, commissions and estimated offering expenses. | |||||||
|
| |||||||
|
Certain insiders may purchase shares in this offering. Because the Company has not entered into any binding agreements or received any commitments to purchase from any insiders, such insiders may elect not to purchase any shares in this offering. | |||||||
|
| |||||||
|
We have granted the underwriters an option for a period of 30 days from the date of this prospectus supplement to purchase up to an additional shares of common stock to cover overallotments at the public offering price, less underwriting discounts and commissions. If the underwriters exercise their option in full, the total underwriting discounts and commissions payable by us will be $ , and the total proceeds to us, before expenses, will be $ . | |||||||
|
| |||||||
|
The underwriters expect to deliver the shares against payment on or about March , 2018, subject to customary closing conditions. | |||||||
|
______________________________ | |||||||
|
Sole Book – Running Manager Oppenheimer & Co. | |||||||
|
Co-Manager National Securities Corporation | |||||||
|
The date of this prospectus supplement is March , 2018. | |||||||
|
| |||||||
|
|
Page |
|
PROSPECTUS SUPPLEMENT |
|
|
ABOUT THIS PROSPECTUS SUPPLEMENT |
S-1 |
|
PROSPECTUS SUPPLEMENT SUMMARY |
S-2 |
|
RISK FACTORS |
S-5 |
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION |
S-16 |
|
USE OF PROCEEDS |
S-17 |
|
PRICE RANGE OF OUR COMMON STOCK |
S-17 |
|
DIVIDEND POLICY |
S-17 |
|
CAPITALIZATION |
S-18 |
|
DILUTION |
S-18 |
|
DESCRIPTION OF COMMON STOCK |
S-19 |
|
UNDERWRITING |
S-20 |
|
LEGAL MATTERS |
S-26 |
|
EXPERTS |
S-26 |
|
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE |
S-26 |
|
WHERE YOU CAN FIND MORE INFORMATION |
S-27 |
|
|
|
|
|
|
|
PROSPECTUS |
|
|
ABOUT THIS PROSPECTUS |
1 |
|
THE COMPANY |
1 |
|
RISK FACTORS |
2 |
|
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION |
2 |
|
USE OF PROCEEDS |
2 |
|
GENERAL DESCRIPTION OF SECURITIES |
3 |
|
DESCRIPTION OF CAPITAL STOCK |
3 |
|
DESCRIPTION OF THE WARRANTS |
4 |
|
PLAN OF DISTRIBUTION |
5 |
|
LEGAL MATTERS |
6 |
|
EXPERTS |
6 |
|
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE |
6 |
|
WHERE YOU CAN FIND MORE INFORMATION |
7 |
ABOUT THIS PROSPECTUS SUPPLEMENT
This document is part of a registration statement that was filed with the Securities and Exchange Commission, or the SEC, using a “shelf” registration process and consists of two parts. The first part is the prospectus supplement, including the documents incorporated by reference herein, which describes the specific terms of this offering. The second part, the accompanying prospectus, including the documents incorporated by reference therein, provides more general information. In general, when we refer only to the prospectus, we are referring to both parts of this document combined. Before you invest, you should carefully read this prospectus supplement, the accompanying prospectus, all information incorporated by reference herein and therein, as well as the additional information described under the heading “Where You Can Find More Information.” These documents contain information you should carefully consider when deciding whether to invest in our common stock.
This prospectus supplement may add, update or change information contained in the accompanying prospectus. To the extent there is a conflict between the information contained in this prospectus supplement and the accompanying prospectus, you should rely on information contained in this prospectus supplement, provided that if any statement in, or incorporated by reference into, one of these documents is inconsistent with a statement in another document having a later date, the statement in the document having the later date modifies or supersedes the earlier statement. Any statement so modified will be deemed to constitute a part of this prospectus only as so modified, and any statement so superseded will be deemed not to constitute a part of this prospectus.
You should rely only on the information contained in this prospectus supplement, the accompanying prospectus, any document incorporated by reference herein or therein, or any free writing prospectuses we may provide to you in connection with this offering. Neither we nor any of the underwriters have authorized anyone to provide you with any different information. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may provide to you. The information contained in this prospectus supplement, the accompanying prospectus, and in the documents incorporated by reference herein or therein is accurate only as of the date such information is presented. Our business, financial condition, results of operations and prospects may have changed since that date.
This prospectus supplement and the accompanying prospectus do not constitute an offer to sell or the solicitation of an offer to buy any securities other than the shares of common stock to which it relates, nor do this prospectus supplement and the accompanying prospectus constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction.
Unless otherwise indicated, information contained in or incorporated by reference into this prospectus concerning our industry and the markets in which we operate, including market position and market opportunity, is based on information from our management’s estimates, as well as from industry publications and research, surveys and studies conducted by third parties. Management estimates are derived from publicly available information, our knowledge of our industry and assumptions based on such information and knowledge, which we believe to be reasonable. However, assumptions and estimates of our future performance, and the future performance of our industry are subject to numerous known and unknown risks and uncertainties, including those described under the heading “Risk Factors” beginning on page S-5 of this prospectus supplement. These and other important factors could result in our estimates and assumptions being materially different from future results. You should read the information contained in, or incorporated by reference into, this prospectus completely and with the understanding that future results may be materially different and worse from what we expect. See the information included under the heading “Cautionary Note Regarding Forward-Looking Information.”
Unless otherwise stated in this prospectus supplement and the accompanying prospectus, references to “Company,” “VolitionRx,” “we,” “us,” or “our” refer to VolitionRx Limited and its wholly-owned subsidiaries. Nucleosomics®, Nu.QTM and Hypergenomics® and their respective logos are trademarks and/or service marks of VolitionRx and its subsidiaries. All other trademarks, service marks and trade names referred to in this prospectus supplement and the accompanying prospectus are the property of their respective owners.
S-1
|
PROSPECTUS SUPPLEMENT SUMMARY |
|
|
|
This prospectus supplement summary discusses the key aspects of the offering and highlights certain information appearing elsewhere in this prospectus supplement, in the accompanying prospectus and in the documents we incorporate by reference herein and therein. However, as this is a summary, it does not contain all of the information that you should consider before deciding to invest in our common stock. You are encouraged to carefully read this entire prospectus, the accompanying prospectus, any free writing prospectus that we have been authorized to use and the documents incorporated by reference herein and in the accompanying prospectus. You should pay special attention to the information provided under the heading “Risk Factors” in this prospectus supplement and in the accompanying prospectus and under the heading “Management’s Discussion and Analysis of Financial Condition and Results of Operations” and our financial statements and the related notes in our Annual Report on Form 10-K for the fiscal year ended December 31, 2017, incorporated by reference herein. |
|
|
|
|
|
Company Overview |
|
|
|
We are a multi-national life sciences company developing simple, easy to use, cost effective blood tests to help diagnose a range of cancers. |
|
|
|
Our tests are based on the science of Nucleosomics®, which is the practice of identifying and measuring nucleosomes in the bloodstream or other bodily fluid – an indication that disease is present. |
|
|
|
The principle behind what we are doing relies on bringing together two main lines of research: the chromosomes of cancer cells differ from those of healthy cells – both in terms of DNA sequence (due to genetic cancer mutations) and in protein structure - due to epigenetic changes. There are chromosome fragments from dead cancer cells circulating in the blood as nucleosomes. Each such circulating nucleosome contains a small (approx. 140bp) fragment of tumor DNA. |
|
|
|
Our Nucleosomics technology exploits the different compositions of circulating nucleosome structures present in the serum of cancer patients to detect and identify cancer diseases. |
|
|
|
We have developed a novel suite of blood assays for epigenetically altered circulating nucleosomes as biomarkers in cancer. Nu.QTM products are simple, low-cost, ELISA platform tests and can incorporate other off patent, low cost ELISA tests in our panels (e.g. CEA, PSA, CA125) for higher accuracy. |
|
|
|
Our diagnostic target in the blood is the same tumor chromosome fragment, but our approach is to test for chromosome protein and nucleic acid changes in intact chromosome fragments by ELISA, rather than chemically extracting, amplifying, and sequencing the ctDNA and discarding the rest of the nucleosome. ELISA is possible because the targets of our tests occur globally across all nucleosomes within a tumor cell, whereas individual ctDNA changes must be identified within the three billion base-pair genomes. This means that the targets of our tests are exponentially more prevalent in circulating blood, and detectable using simple laboratory methods. |
|
|
|
How is Nu.Q different from ctDNA?
When a cancer cell dies, the nuclear components are metabolized into 20 million individual DNA-Nu complexes and released into circulation. A cancer mutation will occur in one of the DNA-Nu complexes. ctDNA sequencing methods (in development) must target that one in 20 million DNA-Nu complexes. Nu.Q targets all 20 million circulating DNA-Nu complexes because nucleosome modifications occur globally. Nu.Q is a simple, low-cost ELISA and can incorporate other ELISA tests in our panels.
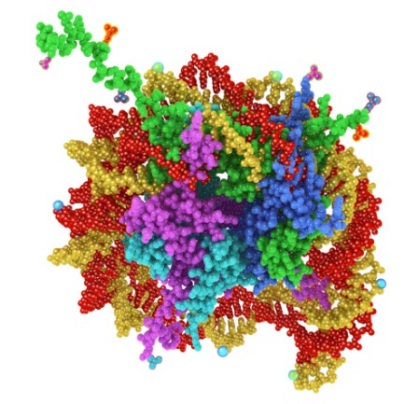
Using our Nucleosomics technology, we have developed 39 epigenetic Nu.Q assays, which are designed to detect the level and structure of nucleosomes in blood. Epigenetics is the science of how genes are switched “on” or “off” in the body’s cells. A major factor controlling the switching “on” and “off” is the structuring of DNA. The DNA in human cells is packaged as protein complexes in a “beads on a string” structure. Each individual protein/DNA “bead” is called a nucleosome. These nucleosomes then form additional structures with increasingly dense packing, culminating in chromosomes containing hundreds of thousands of nucleosomes as depicted in Figure 1 below. |
|
|
S-2
|
Figure 1 – A nucleosome
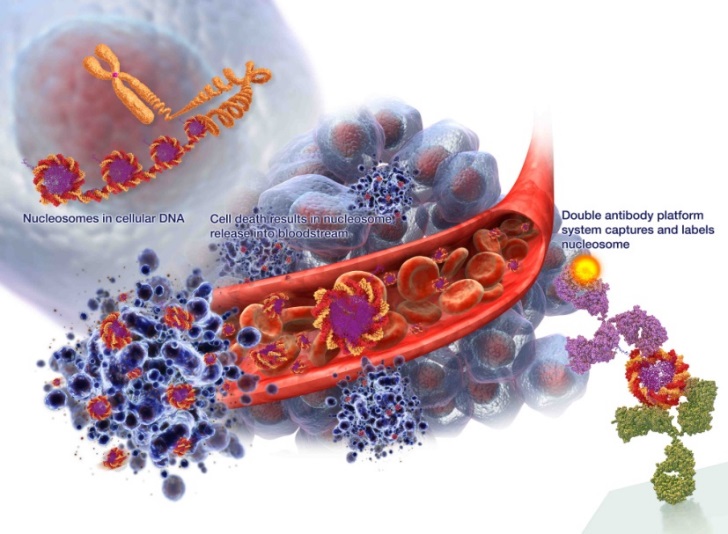
Cancer is characterized by uncontrolled and often rapid cell growth which exceeds the corresponding rate of cell death. When cells die, the DNA fragments into individual nucleosomes which are released into the blood as illustrated in Figure 2 below. The cell debris in the bloodstream is eventually recycled back into the body. When a cancer is present, the number of dying cells can overwhelm the recycling process, leaving the excess fragments, including the nucleosomes, in the blood. Importantly, the structure of nucleosomes is not uniform but subject to immense variety, and nucleosomes in cancer cells have differences in structure from those in healthy cells. |
|
|
|
Figure 2 – Release of nucleosomes into blood
Blood nucleosome levels can be raised in conditions other than cancer, including in auto-immune disease, inflammatory disease, endometriosis, sepsis, and in the immediate aftermath of major trauma (for example following a heart attack, surgery or car accident). Our primary focus is on cancer diagnosis, but we also intend to pursue diagnostic opportunities in other disease areas. |
|
|
|
We have incurred losses since inception, have negative cash flows from operations, and currently have no revenues, and we do not anticipate earning significant revenues until such time as we are able to fully market our intended products. For this reason, our auditors stated in their report on our most recent audited financial statements that our losses and negative cash flow from operations raise substantial doubt that we will be able to continue as a going concern without further financing. See Item 8. Financial Statements and Supplementary Data of our Annual Report on Form 10-K filed with the SEC on March 1, 2018 for a discussion of our ability to continue as a going concern. Our ability to continue as a going concern is dependent upon our ability to successfully accomplish our plan of operations described herein, obtain financing and eventually attain profitable operations. |
|
|
|
Corporate Information |
|
|
|
We are a Delaware corporation. Our executive offices are located at 1 Scotts Road, #24-05 Shaw Centre, Singapore 228208, and our telephone number is +1 (646) 650-1351. We maintain a website at www.volitionrx.com. Our Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, Current Reports on Form 8-K, and all amendments to such reports are available to you free of charge through the Investors section of www.volitionrx.com as soon as practicable after such materials have been electronically filed with, or furnished to, the Securities and Exchange Commission. The information contained on our website is not incorporated by reference into this prospectus. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website. |
|
|
S-3
|
The Offering | ||
|
| ||
|
Issuer: |
|
VolitionRx Limited |
|
Offering Price: |
|
$ per share |
|
|
|
|
|
Common Stock offered by us: |
|
shares (or shares if the underwriters exercise in full their option to purchase additional shares) |
|
|
|
|
|
Common Stock to be outstanding immediately after this offering: |
|
shares (or shares if the underwriters exercise in full their option to purchase additional shares) |
|
|
|
|
|
Option to purchase additional shares: |
|
The underwriters have an option to purchase a maximum of additional shares of common stock from us to cover over-allotments. The underwriters can exercise this option at any time within 30 days from the date of this prospectus supplement. |
|
|
|
|
|
Use of proceeds: |
|
We intend to use the net proceeds from this offering for continued product development, clinical studies, product commercialization, working capital and other general corporate purposes. See the information included under the heading “Use of Proceeds.” |
|
|
|
|
|
Risk factors: |
|
Investing in our common stock involves a high degree of risk. See the information included under the heading “Risk Factors” beginning on page S-5 of this prospectus supplement for a discussion of factors that you should carefully consider before deciding to invest in our common stock. |
|
|
|
|
|
Trading symbol: |
|
Our common stock is currently quoted on the NYSE American under the symbol “VNRX.” |
|
| ||
|
Certain insiders may purchase shares in this offering. Because the Company has not entered into any binding agreements or received any commitments to purchase from any insiders, such insiders may elect not to purchase any shares in this offering. | ||
|
| ||
|
Unless otherwise indicated, the number of shares of our common stock to be outstanding after this offering is based on 26,519,394 shares of our common stock outstanding as of December 31, 2017, and excludes: | ||
|
| ||
|
1,731,680 shares of our common stock issuable upon the exercise of common stock purchase warrants outstanding as of December 31, 2017, with a weighted average exercise price of approximately $2.36 per share; | ||
|
2,939,134 shares of our common stock issuable upon the exercise of stock options outstanding as of December 31, 2017, with an exercise price of approximately $4.09 per share; and | ||
|
829,000 additional shares of common stock reserved for future issuance under our 2015 Stock Incentive Plan, as of December 31, 2017. | ||
|
| ||
|
Unless otherwise indicated, this prospectus reflects and assumes the following: | ||
|
| ||
|
no exercise of the outstanding options and warrants described above; and | ||
|
no exercise by the underwriters of their option to purchase additional shares of our common stock. | ||
|
| ||
S-4
Investing in our common stock involves a high degree of risk. Before making an investment decision, you should carefully consider the risks described below, together with all of the other information included in this prospectus supplement, the accompanying prospectus, and the information incorporated by reference herein and therein.
If any of the risks described below, or those incorporated by reference into this prospectus, actually occur, our business, financial condition or results of operations could suffer. In that case, the trading price of our common stock may decline and you may lose all or part of your investment. The risks and uncertainties we have described are not the only ones we face. Additional risks and uncertainties not presently known to us or that we currently deem immaterial may also affect our business, financial condition and results of operations. Certain statements below are forward-looking statements. See the information included under the heading “Cautionary Note Regarding Forward-Looking Information.”
Risks Associated with our Company
We have not generated any significant revenue since our inception and we may never achieve profitability.
We are a clinical stage company and have incurred losses since our formation. As of December 31, 2017, we have an accumulated total deficit of approximately $55.7 million. As we continue the discovery and development of our future diagnostic products, our expenses are expected to increase significantly. Even as we begin to market and sell our intended products, we expect our losses to continue as a result of ongoing research and development expenses, as well as increased manufacturing, sales and marketing expenses. These losses, among other things, have had and will continue to have an adverse effect on our working capital, total assets and stockholders’ equity. Because of the numerous risks and uncertainties associated with our product development and commercialization efforts, we are unable to predict when we will become profitable, and we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. If we are unable to achieve and then maintain profitability, our business, financial condition and results of operations will be negatively affected and the market value of our common stock will decline.
We may need to raise additional capital in the future. If we are unable to secure adequate funds on terms acceptable to us, we may be unable to execute our plan of operations.
We will require additional capital to fully fund our current strategic plan, which includes successfully commercializing our Nu.Q colorectal cancer pipeline and developing future products. If we incur delays in commencing commercialization of our Nu.Q colorectal cancer pipeline or other future products or in achieving significant product revenue, or if we encounter other unforeseen adverse business developments, we may exhaust our capital resources prior to the commencement of commercialization.
We cannot be certain that additional capital will be available when needed or that our actual cash requirements will not be greater than anticipated. Financing opportunities may not be available to us, or if available, may not be available on favorable terms. The availability of financing opportunities will depend on various factors, such as market conditions and our financial condition and outlook. In addition, if we raise additional funds through the issuance of equity or convertible debt securities, the percentage ownership of our stockholders could be significantly diluted, and these newly-issued securities may have rights, preferences or privileges senior to those of existing stockholders. If we obtain debt financing, a substantial portion of our operating cash flow may be dedicated to the payment of principal and interest on such indebtedness, and the terms of the debt securities issued could impose significant restrictions on our operations. If we are unable to obtain financing on terms favorable to us, we may be unable to execute our plan of operations and we may be required to cease or reduce development or commercialization of any future products, sell some or all of our technology or assets or merge with another entity.
It is difficult to forecast our future performance, which may cause our financial results to fluctuate unpredictably.
Our limited operating history and the rapid evolution of the market for diagnostic products make it difficult for us to predict our future performance. A number of factors, many of which are outside of our control, may contribute to fluctuations in our financial results, such as:
our ability to develop or procure antibodies for clinical use in our future products;
our ability to translate preliminary clinical results to larger prospective symptomatic and screening populations;
the demand for our intended products;
our ability to obtain any necessary financing;
our ability to market and sell our future products;
market acceptance of our future products and technology;
performance of any future strategic business partners;
S-5
our ability to obtain regulatory clearances or approvals;
our success in collecting payments from third-party payors and customers;
changes in technology that may render our future products uncompetitive or obsolete;
competition with other cancer diagnostics companies; and
adverse changes in the healthcare industry.
Our future success depends on our ability to retain our officers and directors, scientists, and other key employees and to attract, retain and motivate qualified personnel.
Our success depends on our ability to attract, retain and motivate highly qualified management and scientific personnel. In particular, we are highly dependent on Cameron Reynolds, our President and Chief Executive Officer, our other officers and directors, scientists and key employees. The loss of any of these persons or their expertise would be difficult to replace and could have a material adverse effect on our ability to achieve our business goals. In addition, the loss of the services of any one of these persons may impede the achievement of our research, development and commercialization objectives by diverting management’s attention to the identification of suitable replacements, if any. There can be no assurance that we will be successful in hiring or retaining qualified personnel and our failure to do so could have a material adverse effect on our business, financial condition and results of operations.
Recruiting and retaining qualified scientific personnel and, in the future, sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among pharmaceutical, biotechnology and diagnostic companies for similar personnel. We also experience competition for the hiring of scientific personnel from universities and research institutions. We do not maintain “key person” insurance on any of our employees. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research, development and commercialization strategies. Our consultants and advisors, however, may have other commitments or employment that may limit their availability to us.
We expect to expand our product development, research and sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We are focused on developing our pipeline for future products. Our efforts will result in significant growth in the number of our consultants, advisors, and employees and the scope of our operations. In order to manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities, and continue to recruit and train additional qualified personnel. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plan or disrupt our operations.
We have limited experience with direct sales and marketing and any failure to build and manage a direct sales and marketing team effectively, or to successfully engage third party providers for such services, could have a material adverse effect on our business.
Our products will require several dynamic and evolving sales models tailored to different worldwide markets, users and products. In 2015, we decided to focus our sales strategy on the clinical in vitro diagnostic device, or IVD, market with the CE Marking of our first product in Europe. Following CE Marking of our first product in Europe we intend to enter the European markets and, following the completion of any necessary regulatory clearances, certain Asian markets. Even when we have received a CE Mark, we must still seek regulatory clearance in other jurisdictions. A failure to obtain these regulatory clearances in other jurisdictions could negatively affect our business. Pending completion of our review of the regulatory environment in the United States, including the effect of recent pronouncements regarding LDTs by the FDA, we may decide to enter the United States market through a CLIA certified laboratory in the United States. We intend to progressively grow to large volumes of tests sold to centralized laboratories and eventually reach the mass diagnostics testing market. The exact nature of the ideal sales strategy will evolve as we continue to develop our intended products and seek entry into the IVD markets. We have limited experience with direct sales and marketing and we currently intend to engage a network of distributors to help commercialize our products worldwide. Any failure to build and manage a direct sales and marketing team effectively, or to successfully engage third party providers for such services, could have a material adverse effect on our business.
There are significant risks involved in building and managing our sales and marketing organization, as well as identifying and negotiating deals with the right sales and distribution partners, including risks related to our ability to:
identify appropriate partners;
negotiate beneficial partnership and distribution agreements;
hire qualified individuals as needed;
generate sufficient leads within our targeted market for our sales force;
S-6
provide adequate training for effective sales and marketing;
protect intellectual property rights;
retain and motivate our direct sales and marketing professionals; and
effectively oversee geographically dispersed sales and marketing teams.
Our failure to adequately address these risks could have a material adverse effect on our ability to increase sales and use of our future products, which would cause our revenues to be lower than expected and harm our results of operations.
Our Second Amended and Restated Certificate of Incorporation exculpates our officers and directors from certain liability to our Company and our stockholders.
Our Second Amended and Restated Certificate of Incorporation contains a provision limiting the liability of our officers and directors for their acts or failures to act, except for acts involving intentional misconduct, fraud or a knowing violation of law. This limitation on liability may reduce the likelihood of derivative litigation against our officers and directors and may discourage or deter our stockholders from suing our officers and directors based upon breaches of their duties to our Company.
We have identified material weaknesses in our internal control over financial reporting that have not yet been remediated, and the failure to address these material weaknesses, or the identification of any others, could impact the reliability of our financial reporting and harm investors’ views of us, which could adversely impact our stock price.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. As defined in Exchange Act Rule 13a-15(f), internal control over financial reporting is a process designed by, or under the supervision of, the principal executive and principal financial officer and effected by the board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and dispositions of assets;
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and/or directors; and
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
We have determined that we have material weaknesses in our internal control over financial reporting as of December 31, 2017. See Item 9A. Controls and Procedures of our Annual Report on Form 10-K filed with the SEC on March 1, 2018 for a complete discussion of these material weaknesses in our internal control over financial reporting and remediation efforts. Although we are undertaking steps to address these material weaknesses, the existence of a material weakness is an indication that there is more than a remote likelihood that a material misstatement of our financial statements will not be prevented or detected in the current or any future period. There can be no assurance that we will be able to fully implement our plans and controls, as further described in Item 9A of our Annual Report on Form 10-K filed with the SEC on March 1, 2018, to address these material weaknesses, or that the plans and controls, if implemented, will be successful in fully remediating these material weaknesses. In addition, we may in the future identify further material weaknesses in our internal control over financial reporting that we have not discovered to date. If we fail to successfully remediate the identified material weaknesses, or we identify further material weaknesses in our internal controls, the market’s confidence in our financial statements could decline and the market price of our common stock could be adversely impacted. Additionally, for so long as we remain as a smaller reporting company, under current rules our accounting firm will not be required to provide an opinion regarding our internal controls over financial reporting.
We have a “going concern” opinion from our auditors, indicating the possibility that we may not be able to continue to operate.
Our independent registered public accountants have expressed substantial doubt about our ability to continue as a going concern. This opinion could materially limit our ability to raise additional funds by issuing new debt or equity securities or otherwise. If we fail to raise sufficient capital when needed, we will not be able to complete our proposed business plan. As a result we may have to liquidate our business and investors may lose their investments. Our ability to continue as a going concern is dependent upon our ability to successfully accomplish our plan of operations described herein, obtain financing and eventually attain profitable operations. Investors should consider our independent registered public accountant’s comments when deciding whether to invest in the Company.
S-7
Our management has broad discretion over the use of our available cash and might not spend available cash in ways that increase the value of your investment.
As of December 31, 2017, we had $10.1 million in combined cash and marketable securities compared to $21.7 million as of December 31, 2016. Our management currently expects to deploy these resources primarily to expand our commercialization activities, to fund our product development efforts and for general corporate and working capital purposes. However, our management has broad discretion to pursue other objectives. You will be relying on the judgment of our management regarding the application and prioritization of our resources. Our management might not apply our cash in ways that increase or permit any return of your investment.
Risks Associated with our Business
Failure to successfully develop, manufacture, market, and sell our future products will have a material adverse effect on our business, financial condition, and results of operations.
We are in the process of developing a suite of diagnostic tests as well as additional products. The successful development and commercialization of our intended products is critical to our future success. Our ability to successfully develop, manufacture, market, and sell our future products is subject to a number of risks, many of which are outside our control. There can be no assurance that we will be able to develop and manufacture products in commercial quantities at acceptable costs, successfully market any products, or generate revenues from the sale of any products. Failure to achieve any of the foregoing would have a material adverse effect on our business, financial condition, and results of operations.
Our business is dependent on our ability to successfully develop and commercialize diagnostic products. If we fail to develop and commercialize diagnostic products, we may be unable to execute our plan of operations.
Our current business strategy focuses on discovering, developing and commercializing diagnostic products. The success of our business will depend on our ability to fully develop and commercialize the diagnostic products in our current development pipeline as well as continue the discovery and development of other diagnostics products. Currently, we are heavily dependent on our Nu.Q colorectal cancer pipeline. The commercial success of our Nu.Q colorectal cancer pipeline will impact our ability to generate revenues.
Prior to commercializing the Nu.Q Colorectal Cancer Screening Triage Test and other diagnostic products, we will be required to undertake time-consuming and costly development activities with uncertain outcomes, including conducting clinical studies and obtaining regulatory clearance or approval in the United States and in Europe. Delays in obtaining approvals and clearances could have material adverse effects on us and our ability to fully carry out our plan of operations. We have limited experience in taking products through these processes and there are considerable risks involved in these activities. The science and methods that we are employing are innovative and complex, and it is possible that our development programs will ultimately not yield products suitable for commercialization or government approval. Products that appear promising in early development may fail to be validated in subsequent studies, and even if we achieve positive results, we may still fail to obtain the necessary regulatory clearances or approvals. Few research and development projects result in commercial products, and perceived viability in early clinical studies often is not replicated in later studies. At any point, we may abandon development of a product, or we may be required to expend considerable resources obtaining additional clinical and nonclinical data, which would adversely impact the timing for generating potential revenue from those products. Further, our ability to develop and launch diagnostic tests is dependent on our receipt of substantial additional funding. If our discovery and development programs yield fewer commercial products than we expect, we may be unable to execute our business plan, and our business, financial condition and results of operations may be adversely affected.
The results of pre-clinical studies and completed clinical trials are not necessarily predictive of future results, and our current product candidates may not have favorable results in later studies or trials which, in turn, could have a material adverse effect on our business.
As described above, we must conduct extensive testing of our product candidates and new indications of our marketed products before we can obtain regulatory approval to market and sell them. Success in pre-clinical studies or completed clinical trials does not ensure that later studies or trials, including continuing pre-clinical studies and large-scale clinical trials, will be successful nor does it necessarily predict future results. Favorable results in early studies or trials may not be repeated in later studies or trials, and product candidates in later stage trials may fail to show acceptable safety and efficacy despite having progressed through earlier trials. We may be required to demonstrate through large, long-term outcome trials that our product candidates are safe and effective for use in a broad population prior to obtaining regulatory approval. The failure of clinical trials to demonstrate the safety and effectiveness of our clinical candidates for the desired indication(s) would preclude the successful development of those candidates for such indication(s), in which event our business, prospects, results of operations and financial condition may be adversely affected.
S-8
Our failure to obtain necessary regulatory clearances or approvals on a timely basis would significantly impair our ability to distribute and market our future products on the clinical IVD market.
We are subject to regulation by the FDA in the United States, the Conformité Européenne in Europe and other regulatory bodies in other countries where we intend to sell our future products. Before we are able to place our intended products in the clinical IVD markets in the United States and Europe, we will be required to obtain clearance or approval of our future products from the FDA and receive a CE Mark, respectively
The European Union has recently adopted regulations that may impose additional requirements to obtain a CE Mark, which could result in delays and further expense, in terms of staff costs to us as compared to the current CE Mark process. The new regulations will require each product submission to be thoroughly audited by Notified Bodies, instead of the current self-certification process. The EU MDR will be fully applicable in 2020 and the EU IVDR will be fully applicable in 2022.
Additionally, even if we receive the required government clearance or approval of our intended products, we are still subject to continuing regulation and oversight. Under the FDA, diagnostics are considered medical devices and are subject to ongoing controls and regulations, including inspections, compliance with established manufacturing practices, device-tracking, record-keeping, advertising, labeling, packaging, and compliance with other standards. The process of complying with such regulations with respect to current and new products can be costly and time-consuming. Failure to comply with these regulations could have a material adverse effect on our business, financial condition, and results of operations. Furthermore, any FDA regulations governing our future products are subject to change at any time, which may cause delays and have material adverse effects on our operations. In Europe, IVD companies are currently able to self-certify that they meet the appropriate regulatory requirements (which is subject to change with the EU MDR and the EU IVDR noted above) but are subject to inspection for enforcement. European national agencies, such as customs authorities and/or the Departments of Health, Industry and Labor, conduct market surveillance to ensure the applicable requirements have been met for products marketed within the European Union.
Reductions or changes in reimbursement policies could limit our ability to sell our products.
Market acceptance and sales of our products will depend, in part, on reimbursement policies and may be affected by healthcare reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which products they will pay for and establish reimbursement levels for those products. To manage healthcare costs, many governments and third-party payors in the United States increasingly scrutinize the pricing of new products and require greater levels of evidence of favorable clinical outcomes and cost-effectiveness before extending coverage. We cannot be sure that reimbursement will be available for our products and, if reimbursement is available, the level of such reimbursement. Reimbursement may impact the demand for, or the price of, our products. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize our future products.
If the marketplace does not accept the products in our development pipeline or any other diagnostic products we might develop, we may be unable to generate sufficient revenue to sustain and grow our business.
Our intended products may never gain significant acceptance in the research or clinical marketplace and therefore may never generate substantial revenue or profits. Physicians, hospitals, clinical laboratories, researchers or others in the healthcare industry may not use our future products unless they determine that they are an effective and cost-efficient means of detecting and diagnosing cancer. If our research and studies do not satisfy providers, payors and others as to the reliability and effectiveness, we may experience reluctance or refusal on the part of the physician to use our future products. In addition, we will need to expend a significant amount of resources on marketing and educational efforts to create awareness of our future products and to encourage their acceptance and adoption. If the market for our future products does not develop sufficiently or the products are not accepted, our revenue potential will be harmed.
The cancer diagnostics market is highly competitive and subject to rapid technological change; accordingly, we will face fierce competition and our intended products may become obsolete.
The cancer diagnostics market is extremely competitive and characterized by evolving industry standards and new product enhancements. Cancer diagnostic tests are technologically innovative and require significant planning, design, development, and testing at the technological, product, and manufacturing process levels. These activities require significant capital commitments and investment. There can be no assurance that our intended products or proprietary technologies will remain competitive following the introduction of new products and technologies by competing companies within the industry. Furthermore, there can be no assurance that our competitors will not develop products that render our future products obsolete or that are more effective, accurate or can be produced at lower costs. There can be no assurance that we will be successful in the face of increasing competition from new technologies or products introduced by existing companies in the industry or by new companies entering the market.
S-9
We expect to face intense competition from companies with greater resources and experience than us, which may increase the difficulty for us to achieve significant market penetration.
The market for cancer diagnostics is intensely competitive, subject to rapid change, and significantly affected by new product introductions and other market activities of industry participants. Our competitors include large multinational corporations and their operating units, including Abbott Laboratories Inc., Cepheid Inc., Philips, GE Healthcare, Siemens, Gen-Probe Incorporated, MDxHealth SA, EpiGenomics AG, Roche Diagnostics, Exact Sciences Corporation and several others. Most of these companies have substantially greater financial, marketing and other resources than we do. Most of these companies are either publicly traded or a division of a publicly traded company, and enjoy several competitive advantages, including:
significantly greater name recognition;
established relationships with healthcare professionals, companies and consumers;
additional lines of products, and the ability to offer rebates or bundle products to offer higher discounts or incentives to gain a competitive advantage;
established supply and distribution networks; and
greater resources for product development, sales and marketing, and intellectual property protection.
Many of these other companies have developed and will continue to develop new products that will compete directly with our future products. In addition, many of our competitors spend significantly greater funds for the research, development, promotion, and sale of new and existing products. These resources may allow them to respond more quickly to new or emerging technologies and changes in consumer requirements. We also face competition in our search for third parties to assist us with sales and marketing and our product candidates, which may negatively impact our ability to enter into favorable sales and marketing arrangements. For all the foregoing reasons, we may not be able to compete successfully against our competitors.
Declining global economic or business conditions may have a negative impact on our business.
Continuing concerns over United States healthcare reform legislation and energy costs, geopolitical issues, the availability and cost of credit and government stimulus programs in the United States and other countries have contributed to increased volatility and diminished expectations for the global economy. These factors, combined with low business and consumer confidence and high unemployment precipitated a global economic slowdown and recession. If the economic climate does not improve or continues to deteriorate, our business, including our access to the Research Use Only, or RUO, or clinical IVD markets for diagnostic tests, could be adversely affected, resulting in a negative impact on our business, financial condition and results of operations.
On June 23, 2016, the United Kingdom held a referendum in which voters approved an exit from the European Union, commonly referred to as “Brexit”. As a result of the referendum, the British government has begun to negotiate the terms of the United Kingdom’s future relationship with the European Union. Although it is unknown what those terms will be, it is possible that there will be greater restrictions on imports and exports between the European Union countries and the United Kingdom and increased regulatory complexities. These changes may adversely affect our ability to market our future products in the United Kingdom which could have an adverse effect on our business, financial condition, and results of operations.
We will rely on third parties to manufacture and supply our intended products. Any problems experienced by these third parties could result in a delay or interruption in the supply of our intended products to our customers, which could have a material negative effect on our business.
We will rely on third parties to manufacture and supply our intended products. The manufacture of our intended diagnostic products will require specialized equipment and utilize complicated production processes that would be difficult, time-consuming and costly to duplicate. If the operations of third party manufacturers are interrupted or if they are unable to meet our delivery requirements due to capacity limitations or other constraints, we may be limited in our ability to fulfill our future sales orders. Any prolonged disruption in the operations of third party manufacturers could have a significant negative impact on our ability to sell our future products, could harm our reputation and could cause us to seek other third party manufacturing contracts, thereby increasing our anticipated development and commercialization costs. In addition, if we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards required by the FDA and with all applicable regulations and guidelines. The delays associated with the verification of a new manufacturer could negatively affect our ability to develop products or receive approval of any products in a timely manner.
S-10
The manufacturing operations of our future third party manufacturers will likely be dependent upon third party suppliers, making us vulnerable to supply shortages and price fluctuations, which could harm our business.
The operations of our future third party manufacturers will likely be dependent upon third party suppliers. A supply interruption or an increase in demand beyond a supplier’s capabilities could harm the ability of our future manufacturers to manufacture our intended products until new sources of supply are identified and qualified.
Reliance on these suppliers could subject us to a number of risks that could harm our business, including:
interruption of supply resulting from modifications to or discontinuation of a supplier’s operations;
delays in product shipments resulting from uncorrected defects, reliability issues, or a supplier’s variation in a component;
a lack of long-term supply arrangements for key components with our suppliers;
inability to obtain adequate supply in a timely manner, or to obtain adequate supply on commercially reasonable terms;
difficulty and cost associated with locating and qualifying alternative suppliers for components in a timely manner;
production delays related to the evaluation and testing of products from alternative suppliers, and corresponding regulatory qualifications;
delay in delivery due to suppliers prioritizing other customer orders over ours;
damage to our brand reputation caused by defective components produced by the suppliers; and
fluctuation in delivery by the suppliers due to changes in demand from us or their other customers.
Any interruption in the supply of components of our future products or materials, or our inability to obtain substitute components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our future customers, which would have an adverse effect on our business.
We will depend on third party distributors in the future to market and sell our future products which will subject us to a number of risks.
We will depend on third party distributors to sell, market, and service our future products in our intended markets. We are subject to a number of risks associated with reliance upon third party distributors including:
lack of day-to-day control over the activities of third party distributors;
third party distributors may not commit the necessary resources to market and sell our future products to our level of expectations;
third party distributors may terminate their arrangements with us on limited or no notice or may change the terms of these arrangements in a manner unfavorable to us; and
disagreements with our future distributors could result in costly and time-consuming litigation or arbitration which we could be required to conduct in jurisdictions with which we are not familiar.
If we fail to establish and maintain satisfactory relationships with our future third party distributors, our revenues and market share may not grow as anticipated, and we could be subject to unexpected costs which could harm our results of operations and financial condition.
If the patents that we rely on to protect our intellectual property prove to be inadequate, our ability to successfully commercialize our future products will be harmed and we may never be able to operate our business profitably.
Our success depends, in large part, on our ability to protect proprietary methods, discoveries and technologies that we develop under the patents and intellectual property laws of the United States, the European Union and other countries, so that we can seek to prevent others from unlawfully using our inventions and proprietary information. We have 17 patent families related to our diagnostic tests, with five patents granted in the United States and four patents granted in the European Union. Additionally, we have 12 patent applications in the name of our subsidiaries pending in the United States and the 13 patent applications in the European Union.
If we are not able to protect our proprietary technology and information, our competitors may use our inventions to develop competing products. We cannot assure you that any of the pending patent applications will result in patents being issued. In addition, due to technological changes that may affect our future products or judicial interpretation of the scope of our patents, our intended products might not, now or in the future, be adequately covered by our patents.
S-11
If third parties assert that we have infringed their patents and proprietary rights or challenge the validity of our patents and proprietary rights, we may become involved in intellectual property disputes and litigation that would be costly, time consuming, and delay or prevent the development or commercialization of our future products.
Our ability to commercialize our intended products depends on our ability to develop, manufacture, market and sell our future products without infringing the proprietary rights of third parties. Third parties may allege that our future products or our methods or discoveries infringe their intellectual property rights. Numerous United States and foreign patents and pending patent applications, which are owned by third parties, exist in fields that relate to our intended products and our underlying methodologies, discoveries and technologies. A third party may sue us for infringing its patent rights.
Our ability to successfully commercialize our intended products depends on our ability to protect our proprietary technology and information. Likewise, we may need to resort to litigation to enforce a patent issued or licensed to us or to determine the scope and validity of third party proprietary rights. In addition, a third party may claim that we have improperly obtained or used its confidential or proprietary information. The cost to us of any litigation or other proceeding relating to intellectual property rights, even if resolved in our favor, could be substantial, and the litigation could divert our management’s attention from other aspects of our business. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of any litigation could limit our ability to continue our operations. Additionally, we cannot be certain of the level of protection, if any, that will be provided by our patents if they are challenged in court, where our competitors may raise defenses such as invalidity, unenforceability or possession of a valid license.
If we are found to infringe upon intellectual property rights of third parties, we might be forced to pay damages, potentially including treble damages. In addition to any damages we might have to pay, a court could require us to stop the infringing activity or obtain a license. Any license required under any patent may not be made available on commercially acceptable terms, if at all. In addition, such licenses are likely to be non-exclusive and, therefore, our competitors may have access to the same technology licensed to us. If we fail to obtain a required license and are unable to design around a patent, we may be unable to effectively market some or all of our future products, which could limit our ability to generate revenue or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations.
If we are unable to protect our trade secrets, we may be unable to protect our interests in proprietary technology, processes and know-how that is not patentable or for which we have elected not to seek patent protection.
In addition to patented technology, we rely upon trade secret protection to protect our interests in proprietary know-how and for processes for which patents are difficult or impossible to obtain or enforce. We may not be able to protect our trade secrets adequately. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors and outside scientific advisors may unintentionally or willfully disclose our information to competitors. Enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets. We rely, in part, on non-disclosure and confidentiality agreements with our employees, consultants and other parties to protect our trade secrets and other proprietary technology. These agreements may be breached and we may not have adequate remedies for any breach. Moreover, others may independently develop equivalent proprietary information, and third parties may otherwise gain access to our trade secrets and proprietary knowledge. Any disclosure of confidential information into the public domain or to third parties could allow our competitors to learn our trade secrets and use the information in competition against us, which could adversely affect our competitive advantage.
Defects in our products may subject us to substantial damages which could materially harm our business or financial condition.
The products we develop could lead to product liability claims based on allegations that one or more of our products contained a design or manufacturing defect which resulted in the failure to detect the disease for which it was designed. A product liability claim could result in substantial damages and be costly and time consuming to defend, either of which could materially harm our business or financial condition. We cannot assure you that our product liability insurance would protect our assets from the financial impact of defending a product liability claim. Any product liability claim brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing insurance coverage in the future.
S-12
Risks Associated with our Common Stock
The market prices and trading volume of our stock may be volatile.
The market price of our common stock is likely to be highly volatile and the trading volume may fluctuate and cause significant price variation to occur. We cannot assure you that the market prices of our common stock will not fluctuate or decline significantly in the future. Some of the factors that could negatively affect the prices of our shares or result in fluctuations in those prices or in trading volume of our common stock could include the following, many of which will be beyond our control:
competition;
comments by securities analysts regarding our business or prospects;
additions or departures of key personnel;
our ability to execute our business plan;
issuance of common stock or other securities;
operating results that fall below expectations;
loss of any strategic relationship;
industry developments;
economic and other external factors; and
period-to-period fluctuations in our financial results.
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price and trading volume of our common stock.
Share ownership by our executive officers and directors make it more difficult for third parties to acquire us or effectuate a change of control that might be viewed favorably by other stockholders.
As of February 27, 2018, our executive officers and directors beneficially owned, in the aggregate, approximately 29.9% of our outstanding shares. As a result, if the executive officers and directors were to oppose a third party’s acquisition proposal for, or a change in control of, the Company, such officers and directors may have sufficient voting power to be able to block or at least delay such an acquisition or change in control from taking place, even if other stockholders would support such a sale or change of control.
Our corporate governance documents, and certain corporate laws applicable to us, could make a takeover attempt, which may be beneficial to our stockholders, more difficult.
Our Board of Directors, or Board, has the power, under our charter documents to:
issue additional shares of common stock without having to obtain stockholder approval for such action;
enable our Board to fill vacant directorships except for vacancies created by the removal of a director;
enable our Board to amend our bylaws without stockholder approval subject to certain exceptions; and
require compliance with an advance notice procedure with regard to business to be brought by a stockholder before an annual or special meeting of stockholders and with regard to the nomination by stockholders of candidates for election as directors.
These provisions may discourage potential acquisition proposals and could delay or prevent a change of control, including under circumstances in which our stockholders might otherwise receive a premium over the market price of our common stock.
We do not expect to pay dividends in the foreseeable future.
We have never declared or paid cash dividends on our common stock. We do not intend to declare dividends for the foreseeable future, as we anticipate that we will reinvest any future earnings in the development and growth of our business. Therefore, investors will not receive any funds unless they sell their common stock, and stockholders may be unable to sell their shares on favorable terms or at all. We cannot assure you of a positive return on investment or that you will not lose the entire amount of your investment in our common stock.
S-13
We may in the future issue additional shares of our common stock which would reduce investors’ ownership interests in the Company and which may cause our stock price to decline.
Our Second Amended and Restated Certificate of Incorporation and amendments thereto authorize the issuance of 100,000,000 shares of common stock, par value $0.001 per share. The future issuance of all or part of our remaining authorized common stock may result in substantial dilution in the percentage of our common stock held by our then existing stockholders. We may value any common stock issued in the future on an arbitrary basis. The issuance of common stock for future services or acquisitions or other corporate actions may have the effect of diluting the percentage ownership of our stockholders and, depending upon the prices at which such shares are sold or issued, on their investment in our common stock and, therefore, could have an adverse effect on any trading market for our common stock.
Future sales of our common stock could depress the market price of our common stock.
Sales of a substantial number of shares of our common stock in the public market or the perception that large sales of our shares could occur, could cause the market price of our common stock to decline or limit our future ability to raise capital through an offering of equity securities.
If equity research analysts do not publish research or reports about our business, or if they do publish such reports but issue unfavorable commentary or downgrade our common stock, the price and trading volume of our common stock could decline.
The trading market for our common stock could be affected by whether and to what extent equity research analysts publish research or reports about us and our business. If one or more equity analysts cover us and publish research reports about our common stock, the price of our stock could decline rapidly if one or more securities analysts downgrade our stock or if those analysts issue or offer unfavorable commentary or cease publishing reports about us. If any of these analysts ceases coverage of us, we could lose visibility in the market, which in turn could cause our common stock price or trading volume to decline and our common stock to be less liquid.
We are a smaller reporting company and we cannot be certain if the reduced disclosure requirements applicable to smaller reporting companies will make our common stock less attractive to investors.
We are currently a “smaller reporting company,” meaning that we are not an investment company, an asset-backed issuer, or a majority-owned subsidiary of a parent company that is not a smaller reporting company and have a public float of less than $75 million measured as of the last business day of our most recently completed second fiscal quarter and annual revenues of less than $50 million during the most recently completed fiscal year. “Smaller reporting companies” are able to provide simplified executive compensation disclosures in their filings; are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting; and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. Decreased disclosures in our SEC filings due to our status as a “smaller reporting company” may make it harder for investors to analyze our results of operations and financial prospects.
Risks Related to this Offering
Purchasers in this offering will experience immediate and substantial dilution in the book value of their investment.
The public offering price of our common stock is substantially higher than the net tangible book value per share of our common stock before giving effect to this offering. Our net tangible book value as of December 31, 2017 was approximately $ , or $ per share. After giving effect to the sale of shares of our common stock at the offering price of $ per share and after deducting the underwriting fees and estimated offering expenses payable by us, if you purchase our common stock in this offering, you will incur immediate substantial dilution of approximately $ per share. Furthermore, if outstanding options or warrants are exercised, you could experience further dilution. See the information included under the heading “Dilution.”
A substantial number of shares of common stock may be sold in the market following this offering, which may depress the market price for our common stock.
Sales of a substantial number of shares of our common stock in the public market following this offering, or the perception that such sales might occur, could cause the market price of our common stock to decline. A substantial number of the outstanding shares of our common stock are, and the shares of common stock sold in this offering upon issuance will be, freely tradable without restriction or further registration under the Securities Act of 1933, as amended, or the Securities Act.
S-14
Upon the completion of this offering, approximately shares of our outstanding common stock beneficially owned by our executive officers, directors and certain of our other existing stockholders will be subject to lock-up agreements with the underwriters of this offering that restrict the sale of shares of our common stock by those parties for a period of 90 days after the date of this prospectus supplement. However, all of the shares sold in this offering and the remaining shares of our common stock outstanding prior to this offering (which include certain shares that are held by our affiliates other than our officers and directors) will not be subject to lock-up agreements with the underwriters and, except to the extent such shares are subject to lock-up agreements, will be freely tradable without restriction under the Securities Act. The market price of our common stock could decline as a result of sales by our stockholders in the market following completion of this offering or the perception that these sales could occur.
We have broad discretion to determine how to use the funds raised in this offering, and may use them in ways that may not enhance our operating results or the price of our common stock.
Our management will have broad discretion over the use of proceeds from this offering, and we could spend the proceeds from this offering in ways our stockholders may not agree with or that do not yield a favorable return. We intend to use the net proceeds of this offering for continued product development, clinical studies, product commercialization, working capital and other general corporate purposes. However, our use of these proceeds may differ substantially from our current plans. If we do not invest or apply the proceeds of this offering in ways that improve our operating results, we may fail to achieve expected financial results, which could have a material adverse effect on our business, financial condition, operating results and cash flow, and which could cause our stock price to decline.
S-15
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
This prospectus supplement, the accompanying prospectus, and the documents incorporated by reference herein and therein, contain forward-looking statements within the meaning of Section 27A of the Securities Act and Section 21 of the Exchange Act. These forward-looking statements are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical fact included in this prospectus supplement, the accompanying prospectus, or the documents incorporated by reference herein or therein, are forward-looking statements. Such statements are typically characterized by terminology such as “may,” “believe,” “will,” “could,” “anticipate,” “expect,” “estimate(s),” “should,” “continue,” “potential,” “plan,” “forecast,” “goal,” “seek,” “intend,” “strategy,” and similar expressions.
Our forward-looking statements are based on our management’s current assumptions and expectations about future events and trends, which affect or may affect our business, strategy, operations or financial performance. Although we believe that these forward-looking statements are based upon reasonable assumptions, they are subject to numerous known and unknown risks and uncertainties and are made in light of information currently available to us. Many important factors, including those described under the heading “Risk Factors” beginning on page S-5 of this prospectus supplement, may adversely and materially affect our results as indicated in forward-looking statements. You should read this prospectus supplement, the accompanying prospectus, and the documents we incorporate by reference herein and therein completely and with the understanding that our actual future results may be materially different and worse from what we expect.
Some significant factors that may impact our estimates and forward-looking statements include:
Our inability to generate any significant revenue or achieve profitability;
Our need to raise additional capital in the future;
Our expectations to expand our product development, research and sales and marketing capabilities could give rise to difficulties in managing our growth;
Our limited experience with direct sales and marketing;
The material weaknesses in our internal control over financial reporting that we have identified;
The possibility that we may not be able to continue to operate, as indicated by the “going concern” opinion from our auditors;
Our ability to successfully develop, manufacture, market, and sell our future products;
Our ability to timely obtain necessary regulatory clearances or approvals to distribute and market our future products;
The acceptance by the marketplace of our future products;
The highly competitive and rapid changing nature of the cancer diagnostics market;
Our reliance on third parties to manufacture and supply our intended products, and such manufacturers’ dependence on third party suppliers;
Our dependence on third party distributors; and
Protection of our patents, intellectual property and trade secrets.
Moreover, we operate in an evolving environment. New risk factors and uncertainties emerge from time to time and it is not possible for our management to predict all risk factors and uncertainties, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. We qualify all of our forward-looking statements by these cautionary statements.
Forward-looking statements speak only as of the date they were made, and, except to the extent required by law or the rules of the NYSE American, we undertake no obligation to update or review any forward-looking statement because of new information, future events or other factors. You should, however, review the risks and uncertainties we describe in the reports we will file from time to time with the SEC after the date of this prospectus supplement. See the information included under the heading “Where You Can Find More Information.”
Forward-looking statements involve risks and uncertainties and are not guarantees of future performance. As a result of the risks and uncertainties described above, the forward-looking statements discussed in this prospectus supplement, the accompanying prospectus, and the documents incorporated by reference herein and therein might not occur and our future results and our performance may differ materially from those expressed in these forward-looking statements due to, but not limited to, the factors mentioned above. Because of these uncertainties, you should not place undue reliance on these forward-looking statements when making an investment decision.
S-16
We estimate that the net proceeds from this offering will be approximately $ million, or approximately $ million if the underwriters fully exercise their option to purchase additional shares, after deducting the underwriting discounts and estimated offering expenses payable by us.
We currently anticipate that we will use the net proceeds received by us for continued product development, clinical studies, product commercialization, working capital and other general corporate purposes. Our expected use of the net proceeds from this offering is based upon our present plans and business condition. As of the date of this prospectus supplement, we cannot predict with certainty all of the particular uses for the net proceeds to be received upon the completion of this offering or the amounts that we will actually spend on the uses set forth above. The amounts and timing of our actual use of proceeds will vary depending on numerous factors, including the factors described under the heading “Risk Factors” beginning on page S-5 of this prospectus supplement. As a result, management will retain broad discretion over the allocation of the net proceeds from this offering, and investors will be relying on the judgment of our management regarding the application of the net proceeds.
PRICE RANGE OF OUR COMMON STOCK
Since February 6, 2015, our common stock has been traded on the NYSE American under the symbol “VNRX.” The following tables set forth the intraday high and low sales prices per share for our common stock for the periods indicated as reported on the NYSE American.
|
Year Ended December 31, 2018 |
High |
Low |
|
First Quarter (through March 7, 2018) |
$4.00 |
$2.53 |
|
Year Ended December 31, 2017 |
High |
Low |
|
First Quarter (Jan. 1 – Mar. 31) |
$5.45 |
$4.01 |
|
Second Quarter (Apr. 1 – Jun. 30) |
$4.20 |
$2.92 |
|
Third Quarter (Jul. 1 – Sept. 30) |
$3.59 |
$2.45 |
|
Fourth Quarter (Oct. 1 – Dec. 31) |
$3.75 |
$2.08 |
|
Year Ended December 31, 2016 |
High |
Low |
|
First Quarter (Jan. 1 – Mar. 31) |
$4.43 |
$3.20 |
|
Second Quarter (Apr. 1 – Jun. 30) |
$4.19 |
$3.05 |
|
Third Quarter (Jul. 1 – Sept. 30) |
$5.86 |
$3.05 |
|
Fourth Quarter (Oct. 1 – Dec. 31) |
$5.39 |
$3.75 |
On March 7, 2018, the last reported sale price of our common stock on the NYSE American was $2.86 per share. On March 7, 2018, there were approximately 168 holders of record of our common stock. The number of holders of record does not include shares held in “street name” through brokers.
We have not previously paid cash dividends on our common stock. It is our current intention to invest our cash flow and earnings in the growth of our business and, therefore, we have no plans to pay cash dividends for the foreseeable future. Investors should not purchase our common stock with the expectation of receiving cash dividends.
S-17
CAPITALIZATION
The following table sets forth our cash, cash equivalents and capitalization, as of December 31, 2017, as follows:
on an actual basis; and
on an as adjusted basis, giving effect to the sale and issuance by us of shares of our common stock in this offering at the public offering price of $ per share, after deducting the underwriting discount and estimated offering expenses payable by us.
You should read this data in conjunction with our historical financial statements and the related notes incorporated by reference in this prospectus supplement and the sections entitled “Management's Discussion and Analysis of Financial Condition and Results of Operations” contained in our most recently filed Annual Report on Form 10-K and other information on file with the SEC that is incorporated by reference in this prospectus supplement and the accompanying prospectus.
|
|
|
As of December 31, 2017 |
||
|
|
|
Actual |
|
As Adjusted |
|
Cash, cash equivalents and short-term investments |
$ |
10,116,263 |
$ |
|
|
Debt obligations |
$ |
(4,666,530) |
$ |
|
|
Stockholders’ Equity: |
$ |
9,957,868 |
$ |
|
|
Common stock, par value $0.001 per share: 100,000,000 shares authorized, 26,519,394 shares issued and outstanding, actual; shares issued and outstanding, as adjusted |
$ |
26,519 |
$ |
|
|
Additional paid-in capital |
$ |
65,774,870 |
$ |
|
|
Accumulated other comprehensive loss |
$ |
(129,343) |
$ |
(129,343) |
|
Accumulated Deficit |
$ |
(55,714,178) |
$ |
|
|
Total stockholders’ equity |
$ |
9,957,868 |
$ |
|
In the table above, the number of shares outstanding after this offering is based on 26,519,394 shares of our common stock outstanding as of December 31, 2017. The number of shares of our common stock outstanding after this offering excludes the following:
1,731,680 shares of our common stock issuable upon the exercise of common stock purchase warrants outstanding as of December 31, 2017, with a weighted average exercise price of approximately $2.36 per share;
2,939,134 shares of our common stock issuable upon the exercise of stock options outstanding as of December 31, 2017, with an exercise price of approximately $4.09 per share;
829,000 additional shares of common stock reserved for future issuance under our 2015 Stock Incentive Plan, as of December 31, 2017; and
any shares issued upon the exercise by the underwriters of the option to purchase up to additional shares of common stock from us to cover over-allotments, if any.
If you invest in our common stock in this offering, your ownership interest will be immediately diluted to the extent of the difference between the public offering price per share of our common stock and the “as adjusted” net tangible book value per share of our common stock upon the closing of this offering.
Net tangible book value per share of our common stock is determined by subtracting our total liabilities from the amount of our total tangible assets (total assets less intangible assets) and then dividing the difference by the number of shares of our common stock deemed to be outstanding at that date. As of December 31, 2017, we had a net tangible book value of $9.4 million, or $0.35 per share of common stock.
Investors purchasing in this offering will incur immediate and substantial dilution. After giving effect to the issuance and sale by us of shares of common stock in this offering at the public offering price of $ per share, and after deducting underwriting discounts and estimated offering expenses payable by us, our as adjusted net tangible book value as of December 31, 2017 would have been approximately $ million, or approximately $ per share. This amount represents an immediate increase in as adjusted net tangible book value of $ per share to our existing stockholders and an immediate dilution in as adjusted net tangible book value of approximately $ per share to new investors purchasing shares of common stock in this offering. Dilution per share to new investors is determined by subtracting as adjusted net tangible book value per share after this offering from the public offering price per share paid by new investors.
S-18
The following table illustrates this dilution on a per share basis:
|
Public offering price per share |
|
|
|
$ |
|
|
Net tangible book value per share as of December 31, 2017 |
$ |
0.35 |
|
|
|
|
Increase in as adjusted net tangible book value per share attributable to this offering |
|
|
|
|
|
|
As adjusted net tangible book value per share after this offering |
|
|
|
|
|
|
Dilution per share to new investors purchasing in this offering |
|
|
|
$ |
|
If the underwriters fully exercise their option to purchase additional shares of our common stock, the as adjusted net tangible book value per share after giving effect to this offering would be $ per share, which amount represents an immediate increase in as adjusted net tangible book value of $ per share of our common stock to existing stockholders, and an immediate dilution to new investors purchasing in this offering of $ per share.
The table above excludes the following shares:
1,731,680 shares of our common stock issuable upon the exercise of common stock purchase warrants outstanding as of December 31, 2017, with a weighted average exercise price of approximately $2.36 per share;
2,939,134 shares of our common stock issuable upon the exercise of stock options outstanding as of December 31, 2017, with an exercise price of approximately $4.09 per share;
829,000 additional shares of common stock reserved for future issuance under our 2015 Stock Incentive Plan, as of December 31, 2017; and
any shares issued upon the exercise by the underwriters of the option to purchase up to additional shares of common stock from us to cover over-allotments, if any.
General
Our authorized capital stock consists of 100,000,000 shares of our common stock, par value $0.001 per share. As of March 7, 2018, there were 26,530,793 shares of our common stock outstanding, which was held of record by 168 stockholders.
Common Stock
Our common stock was quoted on the OTC Bulletin Board beginning on April 12, 2007 under the symbol “SNDC.OB.” Effective October 11, 2011 our symbol was changed to “VNRX.OB” to reflect the Company’s name change. Effective February 6, 2015, we up-listed our common stock onto the NYSE MKT (now NYSE American) and it currently trades under the symbol “VNRX.”
Holders of shares of our common stock are entitled to one vote per share held of record on all matters submitted to a vote of stockholders, including the election of directors. The holders are entitled to receive dividends when, as and if declared by our board of directors, in its discretion, out of funds legally available therefor. In the event of our liquidation, dissolution or winding up, the holders of our common stock are entitled to share ratably in all of our assets remaining after payment of liabilities. The holders of our common stock have no preemptive or other subscription rights, and there are no conversion rights or redemption or sinking fund provisions with respect to such shares. All of the outstanding shares of our common stock are, and the shares of our common stock when issued will be, fully paid and non-assessable.
The material terms and provisions of our common stock are further described under the heading “Description of Capital Stock” starting on page 3 of the accompanying prospectus.
S-19
We entered into an underwriting agreement with the underwriters named below on March , 2018. Oppenheimer & Co. Inc. is acting as the representative of the underwriters. The underwriting agreement provides for the purchase of a specific number of shares of common stock by each of the underwriters. The underwriters’ obligations are several, which means that each underwriter is required to purchase a specified number of shares of common stock, but is not responsible for the commitment of any other underwriter to purchase shares of common stock. Subject to the terms and conditions of the underwriting agreement, each underwriter has severally agreed to purchase the number of shares of common stock set forth opposite its name below:
|
Underwriter |
|
Number of Ordinary Shares |
|
Oppenheimer & Co. Inc. |
|
|
|
National Securities Corporation |
|
|
|
|
|
|
|
Total |
|
|
The underwriters have agreed to purchase all of the shares of common stock offered by this prospectus (other than those covered by the over-allotment option described below) if any are purchased.
The shares of common stock offered hereby are expected to be ready for delivery on or about March , 2018 against payment in immediately available funds.
The underwriters are offering the shares of common stock subject to various conditions and may reject all or part of any order. The representative of the underwriters has advised us that the underwriters propose initially to offer the shares of common stock to the public at the public offering price set forth on the cover page of this prospectus supplement and to dealers at a price less a concession not in excess of $ per share of common stock to brokers and dealers. After the shares of common stock are released for sale to the public, the representative may change the offering price, the concession, and other selling terms at various times.
We have granted the underwriters an over-allotment option. This option, which is exercisable for up to 30 days after the date of this prospectus supplement, permits the underwriters to purchase a maximum of additional shares of common stock from us to cover over-allotments, if any. If the underwriters exercise all or part of this option, they will purchase shares of common stock covered by the option at the public offering price that appears on the cover page of this prospectus supplement, less applicable underwriting discounts and commissions. If this option is exercised in full, the total price to the public will be $ , and the total proceeds to us, before expenses, will be $ . The underwriters have severally agreed that, to the extent the over-allotment option is exercised, they will each purchase a number of additional shares of common stock proportionate to each underwriter’s initial amount reflected in the foregoing table.
The following table provides information regarding the amount of the discounts and commissions to be paid to the underwriters by us, before expenses:
|
|
Per Shares of Common Stock |
|
Total Without Exercise of Over- Allotment Option |
|
Total With Full Exercise of Over- Allotment Option |
|||
|
Public offering price |
$ |
|
|
$ |
|
|
$ |
|
|
Underwriting discounts and commissions(1) |
$ |
|
|
$ |
|
|
$ |
|
|
|
|
|
|
|
|
|
|
|
|
Proceeds, before expenses, to us |
$ |
|
|
$ |
|
|
$ |
|
(1) We have agreed to pay the underwriters a commission of 3% of the aggregate purchase price for shares of common stock sold to officers, directors and certain specified existing holders, or insiders, and 6% of the aggregate purchase price for shares of common stock sold to all other investors; provided that a minimum blended commission of 5% of the aggregate purchase price shall apply so long as at least 50% of such proceeds are raised from sales to investors other than insiders.
Certain insiders may purchase shares in this offering. Because the Company has not entered into any binding agreements or received any commitments to purchase from any insiders, such insiders may elect not to purchase any shares in this offering.
S-20
We estimate that our total expenses of the offering, excluding the estimated underwriting discounts and commissions, will be approximately $ , which includes the fees and expenses for which we have agreed to reimburse the underwriters, including fees and expenses of their counsel (other than expenses related to blue-sky and FINRA filings), up to a maximum aggregate amount of (i) $50,000, in the event that there is no closing of the offering, or (ii) $100,000, in the event that there is a closing of the offering.
We have agreed to indemnify the underwriters against certain liabilities, including liabilities under the Securities Act of 1933.
We and our officers, directors and certain of our other existing stockholders have agreed to a 90-day “lock-up” with respect to our shares of common stock and other of our securities that they beneficially own, including securities that are convertible into shares of common stock and securities that are exchangeable for or exercisable into shares of common stock. This means that, subject to certain exceptions, for a period of 90 days following the date of this prospectus supplement, we and such persons may not offer, sell, pledge or otherwise dispose of these securities without the prior written consent of Oppenheimer & Co. Inc.
The restrictions described in the immediately preceding paragraph do not apply to transfers of shares of common stock or any security convertible into common stock:
as a bona fide gift or gifts;
by will or intestate succession;
to any trust for the direct or indirect benefit of the stockholder or immediate family of the stockholder;
to a charity or educational institution;
the transfer to affiliates, limited partners, members or stockholders of the signatory; or
to any investment fund or other entity controlled or managed by, directly or indirectly, or under common control or management with, any “lock-up” party;
provided that in the case of any transfer or distribution as described in the bullet points above, such transfer shall not involve a disposition for value and each recipient or transferee agrees to be subject to the restrictions described in the immediately preceding paragraph.
Rules of the SEC may limit the ability of the underwriters to bid for or purchase shares of common stock before the distribution of the shares of common stock is completed. However, the underwriters may engage in the following activities in accordance with the rules:
Stabilizing transactions — The representative may make bids or purchases for the purpose of pegging, fixing or maintaining the price of the shares of common stock, so long as stabilizing bids do not exceed a specified maximum.
Over-allotments and syndicate covering transactions — The underwriters may sell more shares of common stock in connection with this offering than the number of shares of common stock that they have committed to purchase. This over-allotment creates a short position for the underwriters. This short sales position may involve either “covered” short sales or “naked” short sales. Covered short sales are short sales made in an amount not greater than the underwriters’ over-allotment option to purchase additional shares of common stock in this offering described above. The underwriters may close out any covered short position either by exercising its over-allotment option or by purchasing shares of common stock in the open market. To determine how they will close the covered short position, the underwriters will consider, among other things, the price of the shares of common stock available for purchase in the open market, as compared to the price at which they may purchase shares of common stock through the over-allotment option. Naked short sales are short sales in excess of the over-allotment option. The underwriters must close out any naked short position by purchasing shares of common stock in the open market. A naked short position is more likely to be created if the underwriters are concerned that, in the open market after pricing, there may be downward pressure on the price of the shares of common stock that could adversely affect investors who purchase shares of common stock in this offering.
Penalty bids — If the representative purchases shares of common stock in the open market in a stabilizing transaction or syndicate covering transaction, it may reclaim a selling concession from the underwriters and selling group members who sold those shares of common stock as part of this offering.
Passive market making — Market makers in the shares of common stock who are underwriters or prospective underwriters may make bids for or purchases of shares of common stock, subject to limitations, until the time, if ever, at which a stabilizing bid is made.
Similar to other purchase transactions, the underwriters’ purchases to cover the syndicate short sales or to stabilize the market price of our shares of common stock may have the effect of raising or maintaining the market price of our shares of common stock or preventing or mitigating a decline in the market price of our shares of common stock. As a result, the price of the shares of common stock may be higher than the price that might otherwise exist in the open market. The imposition of a penalty bid might also have an effect on the price of the shares of common stock if it discourages resales of the shares of common stock.
S-21
Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of the shares of common stock. These transactions may occur on the NYSE American or otherwise. If such transactions are commenced, they may be discontinued without notice at any time.
Relationships
Certain of the underwriters and their affiliates may provide from time to time in the future certain commercial banking, financial advisory, investment banking and other services for us and such affiliates, and for the selling stockholders and their affiliates, in the ordinary course of their business, for which they will receive customary fees and commissions, as applicable, and reimbursement for out-of-pocket expenses. In addition, from time to time, certain of the underwriters and their affiliates may effect transactions for their own account or the account of customers, and hold on behalf of themselves or their customers, long or short positions in our debt or equity securities or loans, and may do so in the future.
Electronic Delivery of Preliminary Prospectus
A prospectus supplement in electronic format may be delivered to potential investors by one or more of the underwriters participating in this offering. The prospectus supplement in electronic format will be identical to the paper version of such prospectus supplement. Other than the prospectus supplement in electronic format, the information on any underwriter’s website and any information contained in any other website maintained by an underwriter is not part of this prospectus supplement, the accompanying prospectus or the registration statement of which this prospectus supplement and the accompanying prospectus form a part.
Notice to Non-U.S. Investors
Other than in the United States and as described below, no action has been taken by us or the underwriters that would permit a public offering of the shares of common stock offered by this prospectus in any jurisdiction where action for that purpose is required. The shares of common stock offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such shares of common stock be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any shares of common stock offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Belgium
The offering is exclusively conducted under applicable private placement exemptions and therefore it has not been and will not be notified to, and this document or any other offering material relating to the shares of common stock has not been and will not be approved by, the Belgian Banking, Finance and Insurance Commission (“Commission bancaire, financière et des assurances/Commissie voor het Bank, Financie en Assurantiewezen”). Any representation to the contrary is unlawful.
Each underwriter has undertaken not to offer sell, resell, transfer or deliver directly or indirectly, any shares of common stock, or to take any steps relating/ancillary thereto, and not to distribute or publish this document or any other material relating to the shares of common stock or to the offering in a manner which would be construed as: (a) a public offering under the Belgian Royal Decree of 7 July 1999 on the public character of financial transactions; or (b) an offering of securities to the public under Directive 2003/71/EC which triggers an obligation to publish a prospectus in Belgium. Any action contrary to these restrictions will cause the recipient and the company to be in violation of the Belgian securities laws.
Canada
This document constitutes an “exempt offering document” as defined in and for the purposes of applicable Canadian securities laws. No prospectus has been filed with any securities commission or similar regulatory authority in Canada in connection with the offer and sale of the securities described herein (the “Securities”). No securities commission or similar regulatory authority in Canada has reviewed or in any way passed upon this document or on the merits of the Securities and any representation to the contrary is an offence.
Canadian investors are advised that this document has been prepared in reliance on section 3A.3 of National Instrument 33-105 Underwriting Conflicts (“NI 33-105”). Pursuant to section 3A.3 of NI 33-105, this document is exempt from the requirement to provide investors with certain conflicts of interest disclosure pertaining to “connected issuer” and/or “related issuer” relationships as would otherwise be required pursuant to subsection 2.1(1) of NI 33-105.
S-22
Resale Restrictions
The offer and sale of the securities in Canada is being made on a private placement basis only and is exempt from the requirement to prepare and file a prospectus under applicable Canadian securities laws. Any resale of Securities acquired by a Canadian investor in this offering must be made in accordance with applicable Canadian securities laws, which may vary depending on the relevant jurisdiction, and which may require resales to be made in accordance with Canadian prospectus requirements, a statutory exemption from the prospectus requirements, in a transaction exempt from the prospectus requirements or otherwise under a discretionary exemption from the prospectus requirements granted by the applicable local Canadian securities regulatory authority. These resale restrictions may under certain circumstances apply to resales of the securities outside of Canada.
Representations of Purchasers
Each Canadian investor who purchases the securities will be deemed to have represented to the issuer and to each dealer from whom a purchase confirmation is received, as applicable, that the investor (i) is purchasing as principal, or is deemed to be purchasing as principal in accordance with applicable Canadian securities laws, for investment only and not with a view to resale or redistribution; (ii) is an “accredited investor” as such term is defined in section 1.1 of National Instrument 45-106 Prospectus Exemptions (“NI 45-106”) or, in Ontario, as such term is defined in section 73.3(1) of the Securities Act (Ontario); and (iii) is a “permitted client” as such term is defined in section 1.1 of National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations.
Taxation and Eligibility for Investment
Any discussion of taxation and related matters contained in this document does not purport to be a comprehensive description of all of the tax considerations that may be relevant to a Canadian investor when deciding to purchase the securities and, in particular, does not address any Canadian tax considerations. No representation or warranty is hereby made as to the tax consequences to a resident, or deemed resident, of Canada of an investment in the securities or with respect to the eligibility of the securities for investment by such investor under relevant Canadian federal and provincial legislation and regulations.
Rights of Action for Damages or Rescission
Securities legislation in certain of the Canadian jurisdictions provides certain purchasers of securities pursuant to an offering memorandum, including where the distribution involves an “eligible foreign security” as such term is defined in Ontario Securities Commission Rule 45-501 Ontario Prospectus and Registration Exemptions and in Multilateral Instrument 45-107 Listing Representation and Statutory Rights of Action Disclosure Exemptions, as applicable, with a remedy for damages or rescission, or both, in addition to any other rights they may have at law, where the offering memorandum, or other offering document that constitutes an offering memorandum, and any amendment thereto, contains a “misrepresentation” as defined under applicable Canadian securities laws. These remedies, or notice with respect to these remedies, must be exercised or delivered, as the case may be, by the purchaser within the time limits prescribed under, and are subject to limitations and defences under, applicable Canadian securities legislation. In addition, these remedies are in addition to and without derogation from any other right or remedy available at law to the investor.
Language of Documents
Upon receipt of this document, each Canadian investor hereby confirms that it has expressly requested that all documents evidencing or relating in any way to the sale of the securities described herein (including for greater certainty any purchase confirmation or any notice) be drawn up in the English language only. Par la réception de ce document, chaque investisseur canadien confirme par les présentes qu’il a expressément exigé que tous les documents faisant foi ou se rapportant de quelque manière que ce soit à la vente des valeurs mobilières décrites aux présentes (incluant, pour plus de certitude, toute confirmation d’achat ou tout avis) soient rédigés en anglais seulement.
S-23
France
Neither this prospectus supplement nor any other offering material relating to the shares of common stock has been submitted to the clearance procedures of the Autorité des marchés financiers in France. The shares of common stock have not been offered or sold and will not be offered or sold, directly or indirectly, to the public in France. Neither this prospectus supplement nor any other offering material relating to the shares of common stock has been or will be: (a) released, issued, distributed or caused to be released, issued or distributed to the public in France; or (b) used in connection with any offer for subscription or sale of the shares of common stock to the public in France. Such offers, sales and distributions will be made in France only: (i) to qualified investors (investisseurs qualifiés) and/or to a restricted circle of investors (cercle restreint d’investisseurs), in each case investing for their own account, all as defined in and in accordance with Articles L.411-2, D.411-1, D.411-2, D.734-1, D.744-1, D.754-1 and D.764-1 of the French Code monétaire et financier; (ii) to investment services providers authorised to engage in portfolio management on behalf of third parties; or (iii) in a transaction that, in accordance with article L.411-2-II-1°-or-2°-or 3° of the French Code monétaire et financier and article 211-2 of the General Regulations (Règlement Général) of the Autorité des marchés financiers, does not constitute a public offer (appel public à l’épargne). Such shares of common stock may be resold only in compliance with Articles L.411-1, L.411-2, L.412-1 and L.621-8 through L.621-8-3 of the French Code monétaire et financier.
Israel
This document does not constitute a prospectus under the Israeli Securities Law, 5728-1968 (the “Securities Law”), and has not been filed with or approved by the Israel Securities Authority. In the State of Israel, this document is being distributed only to, and is directed only at, and any offer of the shares of common stock is directed only at, investors listed in the first addendum to the Israeli Securities Law (the “Addendum”), consisting primarily of joint investment in trust funds, provident funds, insurance companies, banks, portfolio managers, investment advisors, members of the Tel Aviv Stock Exchange, underwriters, venture capital funds, entities with equity in excess of NIS 50 million and “qualified individuals”, each as defined in the Addendum (as it may be amended from time to time), collectively referred to as qualified investors (in each case purchasing for their own account or, where permitted under the Addendum, for the accounts of their clients who are investors listed in the Addendum). Qualified investors will be required to submit written confirmation that they fall within the scope of the Addendum, are aware of the meaning of same and agree to it.
Italy
The offering of the shares of common stock offered hereby in Italy has not been registered with the Commissione Nazionale per la Società e la Borsa (“CONSOB”) pursuant to Italian securities legislation and, accordingly, the shares of common stock offered hereby cannot be offered, sold or delivered in the Republic of Italy (“Italy”) nor may any copy of this prospectus supplement or any other document relating to the shares of common stock offered hereby be distributed in Italy other than to professional investors (operatori qualificati) as defined in Article 31, second paragraph, of CONSOB Regulation No. 11522 of 1 July, 1998 as subsequently amended. Any offer, sale or delivery of the shares of common stock offered hereby or distribution of copies of this prospectus supplement or any other document relating to the shares of common stock offered hereby in Italy must be made:
(a)by an investment firm, bank or intermediary permitted to conduct such activities in Italy in accordance with Legislative Decree No. 58 of 24 February 1998 and Legislative Decree No. 385 of 1 September 1993 (the “Banking Act”);
(b)in compliance with Article 129 of the Banking Act and the implementing guidelines of the Bank of Italy; and
(c)in compliance with any other applicable laws and regulations and other possible requirements or limitations which may be imposed by Italian authorities.
Sweden
This prospectus supplement has not been nor will it be registered with or approved by Finansinspektionen (the Swedish Financial Supervisory Authority). Accordingly, this prospectus supplement may not be made available, nor may the shares of common stock offered hereunder be marketed and offered for sale in Sweden, other than under circumstances which are deemed not to require a prospectus under the Financial Instruments Trading Act (1991: 980).
S-24
Switzerland
The shares of common stock offered pursuant to this prospectus supplement will not be offered, directly or indirectly, to the public in Switzerland and this prospectus supplement does not constitute a public offering prospectus as that term is understood pursuant to art. 652a or art. 1156 of the Swiss Federal Code of Obligations. The company has not applied for a listing of the shares of common stock being offered pursuant to this prospectus supplement on the SWX Swiss Exchange or on any other regulated securities market, and consequently, the information presented in this prospectus supplement does not necessarily comply with the information standards set out in the relevant listing rules. The shares of common stock being offered pursuant to this prospectus supplement have not been registered with the Swiss Federal Banking Commission as foreign investment funds, and the investor protection afforded to acquirers of investment fund certificates does not extend to acquirers of shares of common stock.
Investors are advised to contact their legal, financial or tax advisers to obtain an independent assessment of the financial and tax consequences of an investment in shares of common stock.
United Kingdom/Germany/Norway/The Netherlands
In relation to each Member State of the European Economic Area which has implemented the Prospectus Directive (each, a “Relevant Member State”) an offer to the public of any shares of common stock which are the subject of the offering contemplated by this prospectus supplement may not be made in that Relevant Member State other than the offers contemplated in this prospectus supplement in name(s) of Member State(s) where prospectus will be approved or passported for the purposes of a non-exempt offer once this prospectus supplement has been approved by the competent authority in such Member State and published and passported in accordance with the Prospectus Directive as implemented in name(s) of relevant Member State(s) except that an offer to the public in that Relevant Member State of any shares of common stock may be made at any time under the following exemptions under the Prospectus Directive, if they have been implemented in that Relevant Member State:
(a)to legal entities which are authorized or regulated to operate in the financial markets or, if not so authorized or regulated, whose corporate purpose is solely to invest in securities;
(b)to any legal entity which has two or more of (1) an average of at least 250 employees during the last financial year; (2) a total balance sheet of more than €43,000,000 and (3) an annual net turnover of more than €50,000,000, as shown in its last annual or consolidated accounts;
(c)by the representative to fewer than 100 natural or legal persons (other than qualified investors as defined in the Prospectus Directive); or
(d)in any other circumstances falling within Article 3(2) of the Prospectus Directive, provided that no such offer of shares of common stock shall result in a requirement for the publication by the company or any underwriter of a prospectus pursuant to Article 3 of the Prospectus Directive.
For the purposes of this provision, the expression an “offer to the public” in relation to any shares of common stock in any Relevant Member State means the communication in any form and by any means of sufficient information on the terms of the offer and any shares of common stock to be offered so as to enable an investor to decide to purchase any shares of common stock, as the same may be varied in that Member State by any measure implementing the Prospectus Directive in that Member State and the expression “Prospectus Directive” means Directive 2003/71/EC and includes any relevant implementing measure in each Relevant Member State.
Each underwriter has represented, warranted and agreed that:
(a)it has only communicated or caused to be communicated and will only communicate or cause to be communicated any invitation or inducement to engage in investment activity (within the meaning of section 21 of the Financial Services and Markets Act 2000 (the FSMA)) received by it in connection with the issue or sale of any shares of common stock in circumstances in which section 21(1) of the FSMA does not apply to the company; and
(b)it has complied with and will comply with all applicable provisions of the FSMA with respect to anything done by it in relation to the shares of common stock in, from or otherwise involving the United Kingdom.
S-25
Certain legal matters relating to this offering will be passed upon for us by Stradling Yocca Carlson & Rauth, P.C., Newport Beach, California. Certain legal matters relating to this offering will be passed upon for the underwriters by Mintz, Levin, Cohn, Ferris, Glovsky and Popeo P.C., New York, New York.
Sadler, Gibb & Associates, LLC, an independent registered public accounting firm, has audited our financial statements as of December 31, 2017 and 2016 included in our Annual Report on Form 10-K for the year ended December 31, 2017, as set forth in their report, which is incorporated by reference in this prospectus. Our financial statements are incorporated by reference in reliance on Sadler, Gibb & Associates, LLC’s report, given on their authority as experts in accounting and auditing.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate” into this prospectus information that we file with the SEC in other documents. This means that we can disclose important information to you by referring to other documents that contain that information. Any information that we incorporate by reference into this prospectus is considered part of this prospectus.
Information contained in this prospectus and information that we file with the SEC in the future and incorporate by reference in this prospectus automatically modifies and supersedes previously filed information, including information in previously filed documents or reports that have been incorporated by reference in this prospectus, to the extent the new information differs from or is inconsistent with the old information. Any statement so modified will be deemed to constitute a part of this prospectus only as so modified, and any statement so superseded will be deemed not to constitute a part of this prospectus.
We incorporate by reference, as of their respective dates of filing, the documents listed below that we have filed with the SEC and any additional documents that we may file in the future with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, including any documents filed after the date of the initial registration statement of which this prospectus is a part until the offering of the security covered by this prospectus has been completed, other than, in each case, documents or information deemed to have been “furnished” and not “filed” in accordance with SEC rules:
our Annual Report on Form 10-K for the year ended December 31, 2017, as filed with the SEC on March 1, 2018;
the description of our common stock contained in our registration statement on Form 8-A as filed with the SEC on February 3, 2015, as updated or amended in any amendment or report filed for such purpose.
We hereby undertake to provide without charge to each person, including any beneficial owner, to whom this prospectus is delivered, upon written or oral request, a copy of any or all documents that are incorporated by reference into this prospectus, but not delivered with the prospectus, other than exhibits to such documents unless such exhibits are specifically incorporated by reference into the documents that this prospectus incorporates. To request such materials, please contact Mr. Rodney Rootsaert, our Corporate Secretary, at c/o Corporate Secretary, VolitionRx Limited, 1 Scotts Road, #24-05 Shaw Centre, Singapore, 228208, by email at notice@volitionrx.com, or by facsimile at +32 8172 5651. These documents are also available free of charge through the Investors section on our website at http://www.volitionrx.com as soon as practicable after such materials have been electronically filed with, or furnished to, the SEC.
You should rely only on the information contained in this prospectus, in any accompanying prospectus supplement, or in any document incorporated by reference herein or therein. We have not authorized anyone to provide you with any different information. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may provide to you. The information contained in this prospectus, in any applicable prospectus supplement, and in the documents incorporated by reference herein or therein is accurate only as of the date such information is presented. Our business, financial condition, results of operations and prospects may have changed since that date.
S-26
WHERE YOU CAN FIND MORE INFORMATION
We file annual, quarterly and current reports and other information with the SEC. You may read and copy any document we file at the SEC’s Public Reference Room located at 100 F Street, N.E., Washington, D.C. 20549. You may obtain information on the operation of the public reference room by calling the SEC at 1-800-SEC-0330. Our filings with the SEC also are available from the SEC’s internet site at http://www.sec.gov, which contains reports, proxy and information statements, and other information regarding issuers that file electronically.
This prospectus supplement and the accompanying prospectus are part of a registration statement that we filed with the SEC. As permitted by SEC rules, this prospectus supplement and the accompanying prospectus form a part of the registration statement, but do not contain all of the information that is included in the registration statement. The registration statement contains more information regarding us and our securities, including certain exhibits. You can obtain a copy of the registration statement from the SEC at the address listed above or from the SEC’s website.
S-27
$50,000,000

VOLITIONRX LIMITED
1 Scotts Road
#24-05 Shaw Centre
Singapore 228208
+1 (646) 650-1351
Common Stock
Preferred Stock
Warrants
_______________________
We may, from time to time in one or more offerings, sell up to $50,000,000 in the aggregate, inclusive of any exercise price thereof, of:
shares of our common stock;
shares of our preferred stock;
warrants to purchase shares of our common stock and/or preferred stock; or
any combination of the foregoing.
This prospectus provides a general description of the securities we may offer. The prospectus supplement may also add, update or change information in this prospectus. You should read this prospectus and any prospectus supplement, as well as the documents incorporated by reference or deemed to be incorporated by reference herein or therein, carefully before you invest in any of the securities offered pursuant to this prospectus. This prospectus may not be used to offer or sell our securities unless accompanied by a prospectus supplement relating to the offered securities.
These securities may be sold directly by us, through dealers or agents designated from time to time, to or through underwriters or dealers or through a combination of these methods on a continuous or delayed basis. For additional information on the methods of sale, you should refer to the section entitled “Plan of Distribution” in this prospectus. We will describe the plan of distribution for any particular offering of our securities in a prospectus supplement. If any agents, underwriters or dealers are involved in the sale of any securities with respect to which this prospectus is being delivered, we will set forth in a prospectus supplement the names of such agents or underwriters and any applicable fees, commissions, discounts and over-allotment options. We will also set forth in a prospectus supplement the price to the public of such securities and the net proceeds that we expect to receive from such sale.
Our common stock is currently quoted on the NYSE MKT under the symbol “VNRX.”
As of September 3, 2015, the aggregate market value of our outstanding common stock held by non-affiliates, or public float, was approximately $47,028,724, based on 18,059,715 shares of outstanding common stock, of which approximately 5,175,133 shares were held by affiliates, and a price of $3.65 per share, which was the last reported sale price of our common stock on the NYSE MKT on such date. We have not offered any securities pursuant to General Instruction I.B.6 of Form S-3 during the prior 12 calendar month period that ends on and includes the date of this prospectus. Pursuant to General Instruction I.B.6 of Form S-3, in no event will we sell securities registered on this registration statement in a public primary offering with a value exceeding more than one-third of our public float in any 12-month period so long as our public float remains below $75.0 million.
INVESTING IN THE SECURITIES WE MAY OFFER INVOLVES VARIOUS RISKS. WE STRONGLY RECOMMEND THAT YOU READ CAREFULLY THE RISKS WE DESCRIBE IN THIS PROSPECTUS AS WELL AS IN ANY ACCOMPANYING PROSPECTUS SUPPLEMENT AND THE RISK FACTORS IN OUR MOST CURRENT REPORTS FILED WITH THE SECURITIES AND EXCHANGE COMMISSION. FOR A FULLER UNDERSTANDING OF THE RISKS AND UNCERTAINTIES THAT WE FACE, SEE THE SECTION ENTITLED “RISK FACTORS” ON PAGE 2.
NEITHER THE SECURITIES AND EXCHANGE COMMISSION NOR ANY STATE SECURITIES COMMISSION HAS APPROVED OR DISAPPROVED OF THESE SECURITIES OR PASSED UPON THE ADEQUACY OR ACCURACY OF THIS PROSPECTUS. ANY REPRESENTATION TO THE CONTRARY IS A CRIMINAL OFFENSE.
The date of this prospectus is September 18, 2015
TABLE OF CONTENTS
ABOUT THIS PROSPECTUS1
THE COMPANY1
RISK FACTORS2
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION2
USE OF PROCEEDS2
GENERAL DESCRIPTION OF SECURITIES3
DESCRIPTION OF CAPITAL STOCK3
DESCRIPTION OF THE WARRANTS4
PLAN OF DISTRIBUTION5
LEGAL MATTERS6
EXPERTS6
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE6
WHERE YOU CAN FIND MORE INFORMATION7
ABOUT THIS PROSPECTUS
This prospectus is part of a registration statement that we filed with the Securities and Exchange Commission, or the SEC, using a “shelf” registration process. Under this shelf registration process, we may from time to time offer and sell any combination of the securities described in this prospectus in one or more offerings with an aggregate initial offering price not to exceed $50,000,000. We have provided to you in this prospectus a general description of the securities we may offer. Each time we sell any of our securities under this prospectus, we will, to the extent required by law, provide a prospectus supplement that will contain specific information about the terms of the offering.
We may add, update or change any of the information contained in this prospectus or in any accompanying prospectus supplement we may authorize to be delivered to you. To the extent there is a conflict between the information contained in this prospectus and any accompanying prospectus supplement, you should rely on the information in the prospectus supplement, provided that if any statement in one of these documents is inconsistent with a statement in another document having a later date-for example, a document incorporated by reference in this prospectus or any prospectus supplement-the statement in the document having the later date modifies or supersedes the earlier statement. Any statement so modified will be deemed to constitute a part of this prospectus only as so modified, and any statement so superseded will be deemed not to constitute a part of this prospectus. This prospectus, together with any accompanying prospectus supplement, includes all material information relating to an offering pursuant to this registration statement.
You should rely only on the information contained in this prospectus, in any accompanying prospectus supplement, or in any document incorporated by reference herein or therein. We have not authorized anyone to provide you with any different information. We take no any responsibility for, and can provide no assurance as to the reliability of, any other information that others may provide to you. The information contained in this prospectus, in any applicable prospectus supplement, and in the documents incorporated by reference herein or therein is accurate only as of the date such information is presented. Our business, financial condition, results of operations and prospects may have changed since that date.
This prospectus and any accompanying prospectus supplement does not constitute an offer to sell or the solicitation of an offer to buy any securities other than the registered securities to which they relate, nor does this prospectus and any accompanying prospectus supplement constitute an offer to sell or the solicitation of an offer to buy securities in any jurisdiction to any person to whom it is unlawful to make such offer or solicitation in such jurisdiction. This prospectus may not be used to offer or sell our securities unless accompanied by a prospectus supplement relating to the offered securities.
This prospectus does not contain all of the information included in the registration statement. For a more complete understanding of the offering of the securities, you should refer to the registration statement, including its exhibits. The registration statement containing this prospectus, including the exhibits to the registration statement, provides additional information about us and the securities offered pursuant to this prospectus. The registration statement, including the exhibits, can be read on the SEC’s website or at the SEC’s offices mentioned under the heading “Where You Can Find More Information.”
We may sell the securities to or through underwriters, dealers or agents or directly to purchasers. We and our agents reserve the sole right to accept or reject in whole or in part any proposed purchase of securities. The prospectus supplement, which we will provide to you each time we offer securities, will set forth the names of any underwriters, dealers or agents involved in the sale of the securities, and any applicable fee, commission or discount arrangements with them. See “Plan of Distribution.”
Unless we state otherwise or the context indicates otherwise, references to the “Company”, “VolitionRx”, “we”, “us”, and “our” in this prospectus refer to VolitionRx Limited and its subsidiaries. Our fiscal year ends on December 31 of each calendar year. Nucleosomics®, NuQ® and HyperGenomics® and their respective logos are trademarks and/or service marks of VolitionRx Limited and its subsidiaries. All other trademarks, service marks and trade names referred to in this prospectus are the property of their respective owners.
We are a clinical stage life sciences company focused on developing blood-based diagnostic tests that meet the need for accurate, fast, inexpensive and scalable tests for detecting and diagnosing cancer and other diseases. We have developed twenty blood assays to date that can be used individually or in combination to generate a profile which forms the basis of a blood test for a particular cancer or disease. We intend to commercialize our products in the future through various channels within the European Union, the United States and eventually throughout the rest of the world.
1
We do not anticipate earning significant revenues until such time as we are able to fully market our intended products on the clinical in-vitro diagnostics, or IVD, market. For this reason, our auditors stated in their report on our most recent audited financial statements that our losses and negative cash flow from operations raise substantial doubt that we will be able to continue as a going concern without further financing. Our ability to continue as a going concern is dependent upon our ability to successfully accomplish our plan of operations described herein, obtain financing and eventually attain profitable operations.
We are a Delaware corporation. Our executive offices are located at 1 Scotts Road, #24-05 Shaw Centre, Singapore 228208, and our telephone number is +1 (646) 650-1351. We maintain a website at www.volitionrx.com. The information contained on our website is not incorporated by reference into this prospectus. We have included our website address only as an inactive textual reference and do not intend it to be an active link to our website.
Before making an investment decision, you should carefully consider the risks described under “Risk Factors” in the applicable prospectus supplement and in our most recent Annual Report on Form 10-K, or any updates in our Quarterly Reports on Form 10-Q, together with all of the other information appearing in this prospectus or incorporated by reference into this prospectus and any applicable prospectus supplement, in light of your particular investment objectives and financial circumstances. The risks so described are not the only risks facing our company. Additional risks not presently known to us or that we currently deem immaterial may also impair our business operations. Our business, financial condition or results of operations could be materially adversely affected by any of these risks. The trading price of our securities could decline due to any of these risks, and you may lose all or part of your investment.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING INFORMATION
This prospectus and the documents incorporated by reference herein include forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”) and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”). All statements other than statements of historical fact contained in this prospectus, including statements regarding estimates, future events, our future financial performance, business strategy and plans and objectives of management for future operations, including with respect to us specifically and the cancer diagnostics industry in general, are forward-looking statements. We have attempted to identify estimates and forward-looking statements by terminology including “anticipates,” “believes,” “can,” “continue,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “should,” or “will” or the negative of these terms or other comparable terminology. Although we do not make estimates or forward-looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy. Our estimates and forward-looking statements are based on our current assumptions and expectations about future events and trends, which affect or may affect our business, strategy, operations or financial performance. These statements are only predictions and involve known and unknown risks, uncertainties and other factors, which may cause our or our industry’s actual results, levels of activity, performance or achievements to vary from those expressed or implied by these estimates and forward-looking statements.
Factors that could cause or contribute to such differences in results and outcomes include, but are not limited to, those discussed under the section entitled “Risk Factors” in this prospectus and in any documents incorporated by reference herein. Readers should carefully review this information as well as other risks and uncertainties described in other filings with the SEC that we may make after the filing date of this prospectus.
Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time and it is not possible for us to predict all risk factors, nor can we address the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause our actual results to differ materially from those contained in any estimates or forward-looking statements. All estimates and forward-looking statements speak only as of the date they were made, and, except to the extent required by applicable law or regulation, we undertake no obligation to update or to review any estimate and/or forward-looking statement. In light of these risks and uncertainties, we cannot assure you that the estimates or forward-looking statements contained in this prospectus will in fact occur. You should not place undue reliance on these estimates and forward-looking statements.
We intend to use the net proceeds we receive from the sale of our securities offered by us hereby for working capital and other general corporate purposes. We may set forth additional information regarding the use of proceeds from the sale of securities we offer under this prospectus in a prospectus supplement relating to the specific offering. We have not determined the amount of net proceeds to be used specifically for the foregoing purposes. As a result, our management will have broad discretion in the allocation of the net proceeds.
2
GENERAL DESCRIPTION OF SECURITIES
We, directly or through agents, dealers or underwriters designated from time to time, may offer, issue and sell, together or separately, in one or more offerings, up to $50,000,000 in the aggregate, inclusive of any exercise price thereof, of:
shares of our common stock, par value $0.001 per share;
shares of our preferred stock, par value $0.001 per share;
warrants to purchase shares of our common stock and/or preferred stock; or
any combination of the foregoing, either individually or as units consisting of one or more of the foregoing, each on terms to be determined at the time of sale.
The common stock, the preferred stock and the warrants are collectively referred to herein as the securities. This prospectus provides you with a general description of the securities we may offer. Each time we offer securities, we will provide you with a prospectus supplement that describes the specific amounts, prices and terms of the securities we offer. The securities involve various risks that we will describe in the section entitled “Risk Factors” that will be included in each prospectus supplement.
Common Stock
We have authority under our amended and restated certificate of incorporation to issue up to 100,000,000 shares of our common stock, par value $0.001 per share. As of September 3, 2015, there were 18,059,715 shares of our common stock issued and outstanding.
Holders of shares of our common stock are entitled to one vote per share held of record on all matters submitted to a vote of stockholders, including the election of directors. The holders are entitled to receive dividends when, as and if declared by our board of directors, in its discretion, out of funds legally available therefor, subject to preferences that may be applicable to any outstanding shares of our preferred stock. In the event of our liquidation, dissolution or winding up, the holders of our common stock are entitled to share ratably in all of our assets remaining after payment of liabilities and after payment of any preferential amounts to which holders of shares of any series of our preferred stock that may be outstanding in the future, may be entitled. The holders of our common stock have no preemptive or other subscription rights, and there are no conversion rights or redemption or sinking fund provisions with respect to such shares. All of the outstanding shares of our common stock are, and the shares of our common stock when issued will be, fully paid and non-assessable.
Preferred Stock
We have authority under our restated certificate of incorporation, as amended, to issue up to 1,000,000 shares of our preferred stock, par value $0.001 per share, all of which are presently undesignated. As of September 3, 2015, there were no shares of our preferred stock issued and outstanding.
Our board of directors may from time to time issue the undesignated preferred stock in one or more series and, in connection with the creation of each such series, fix the number of shares of such series and the designations, powers, preferences, rights, qualifications, limitations, and restrictions of such series, to the fullest extent permitted under the Delaware General Corporation Law. Any preferred stock issued by us in the future may decrease the amount of earnings and assets available for distribution to holders of our common stock or adversely affect the rights and power, including voting rights, of the holders of our common stock without any further vote or action by our stockholders. In addition, the rights of holders of our common stock will be subject to, and may be adversely affected by, the rights of the holders of any preferred stock that may be issued by us in the future.
3
DESCRIPTION OF THE WARRANTS
We may issue warrants to purchase shares of our common stock and/or our preferred stock. The warrants may be issued independently or together with shares of our common stock and/or our preferred stock and may be attached to or separate from the shares of our common stock and/or our preferred stock. The warrants are to be issued under warrant agreements to be entered into between us and/or a bank or trust company, as warrant agent, all as shall be set forth in the prospectus supplement relating to warrants being offered pursuant to such prospectus supplement. The following description of the warrants will apply to the warrants offered by this prospectus unless we provide otherwise in the applicable prospectus supplement. The applicable prospectus supplement for a particular series of warrants may specify different or additional terms.
Warrants
The applicable prospectus supplement will describe the following terms of warrants offered:
the title of the warrants;
the securities for which the warrants are exercisable;
the price or prices at which the warrants will be issued;
the provisions, if any, for changes to or adjustments in the exercise price;
the provisions, if any, for call rights or put rights relating to the warrants or the underlying securities;
the date on which the right to exercise the warrants shall commence and the date on which the right will expire;
if applicable, the number of warrants issued with each share of our common stock and/or our preferred stock;
if applicable, the date on and after which the warrants and the related common stock and/or preferred stock will be separately transferable;
a discussion of any material federal income tax consequences of holding or exercising the warrants; and
any other terms of the warrants, including terms, procedures and limitations relating to the exchange and exercise of the warrants.
Holders of warrants will not be entitled, by virtue of being such holders, to vote, consent, receive dividends, receive notice as stockholders with respect to any meeting of stockholders for the election of our directors or any other matter, or to exercise any rights whatsoever as our stockholders.
The exercise price payable and the number of shares of our common stock and/or our preferred stock purchasable upon the exercise of each warrant will be subject to adjustment in certain events, including the issuance of a stock dividend to holders of our common stock and/or our preferred stock or a stock split, reverse stock split, combination, subdivision or reclassification of our common stock and/or our preferred stock. In lieu of adjusting the number of shares of our common stock and/or our preferred stock purchasable upon exercise of each warrant, we may elect to adjust the number of warrants. No fractional shares will be issued upon exercise of the warrants, but we will pay the cash value of any fractional shares otherwise issuable. Notwithstanding the foregoing, in case of any consolidation, merger, or sale or conveyance of our property as an entirety or substantially as an entirety, the holder of each outstanding warrant shall have the right to the kind and amount of shares of stock and other securities and property, including cash, receivable by a holder of the number of shares of our common stock and/or our preferred stock into which the warrant was exercisable immediately prior to such transaction.
Exercise of Warrants
Each warrant will entitle the holder to purchase for cash such shares of our common stock and/or our preferred stock at such exercise price as shall be in each case be set forth in, or be determinable as set forth in, the prospectus supplement relating to the warrants offered thereby. Warrants may be exercised at any time up to the close of business on the expiration date set forth in the prospectus supplement relating to the warrants offered thereby. After the close of business on the expiration date, unexercised warrants will become void.
The warrants may be exercised as set forth in the prospectus supplement relating to the warrants offered. Upon receipt of payment and the warrant certificate properly completed and duly executed at the corporate trust office of the warrant agent or any other office indicated in the prospectus supplement, we will, as soon as practicable, forward the shares of our common stock and/or our preferred stock purchasable upon such exercise. If less than all of the warrants represented by such warrant certificate are exercised, a new warrant certificate will be issued for the remaining warrants.
4
PLAN OF DISTRIBUTION
We may sell our securities from time to time in any manner permitted by the Securities Act of 1933, as amended, or the Securities Act, including any one or more of the following ways:
through agents;
to or through underwriters;
to or through broker-dealers (acting as agent or principal);
in “at the market” offerings, within the meaning of Rule 415(a)(4) of the Securities Act, to or through a market maker or into an existing trading market, on an exchange or otherwise; and/or
directly to purchasers, through a specific bidding or auction process or otherwise.
The securities may be sold at a fixed price or prices, which may be changed, at market prices prevailing at the time of sale, at prices relating to the prevailing market prices or at negotiated prices.
Offers to purchase offered securities may be solicited by agents designated by us from time to time. Any agent involved in the offer or sale of the offered securities in respect of which this prospectus is delivered will be named, and any commissions payable by us will be set forth, in the applicable prospectus supplement. Unless otherwise set forth in the applicable prospectus supplement, any agent will be acting on a reasonable best efforts basis for the period of its appointment. Any agent may be deemed to be an underwriter, as that term is defined in the Securities Act, of the offered securities so offered and sold.
We will set forth in a prospectus supplement the terms of the offering of our securities, including:
the name or names of any agents, underwriters or dealers;
the purchase price of our securities being offered and the proceeds we will receive from the sale;
any over-allotment options under which underwriters may purchase additional securities from us;
any agency fees or underwriting discounts and commissions and other items constituting agents’ or underwriters’ compensation;
the public offering price;
any discounts or concessions allowed or reallowed or paid to dealers; and
any securities exchanges on which such securities may be listed.
If offered securities are sold to the public by means of an underwritten offering, either through underwriting syndicates represented by managing underwriters or directly by the managing underwriters, we will execute an underwriting agreement with an underwriter or underwriters, and the names of the specific managing underwriter or underwriters, as well as any other underwriters, will be set forth in the applicable prospectus supplement. In addition, the terms of the transaction, including commissions, discounts and any other compensation of the underwriters and dealers, if any, will be set forth in the applicable prospectus supplement, which prospectus supplement will be used by the underwriters to make resales of the offered securities. If underwriters are utilized in the sale of the offered securities, the offered securities will be acquired by the underwriters for their own account and may be resold from time to time in one or more transactions, including:
transactions on the NYSE MKT or any other organized market where the securities may be traded;
in the over-the-counter market;
in negotiated transactions; or
under delayed delivery contracts or other contractual commitments.
We may grant to the underwriters options to purchase additional offered securities to cover over-allotments, if any, at the public offering price with additional underwriting discounts or commissions, as may be set forth in the applicable prospectus supplement. If we grant any over-allotment option, the terms of the over-allotment option will be set forth in the applicable prospectus supplement.
We may authorize agents or underwriters to solicit offers by certain types of institutional investors to purchase securities from us at the public offering price set forth in the prospectus supplement pursuant to delayed delivery contracts providing for payment and delivery on a specified date in the future. We will describe the conditions to these contracts and the commissions we must pay for solicitation of these contracts in the prospectus supplement.
5
We may indemnify agents, underwriters and dealers against specified liabilities, including liabilities incurred under the Securities Act, or to contribution by us to payments they may be required to make in respect of such liabilities. Agents, underwriters or dealers, or their respective affiliates, may be customers of, engage in transactions with or perform services for us or our respective affiliates, in the ordinary course of business.
Unless otherwise specified in the applicable prospectus supplement, each class or series of securities will be a new issue with no established trading market, other than our common stock, which is traded on the NYSE MKT. We may elect to list any other class or series of securities on any exchange and, in the case of our common stock, on any additional exchange. However, unless otherwise specified in the applicable prospectus supplement, we will not be obligated to do so. It is possible that one or more underwriters may make a market in a class or series of securities, but the underwriters will not be obligated to do so and may discontinue any market making at any time without notice. We cannot give any assurance as to the liquidity of the trading market for any of the offered securities.
Any underwriter may engage in over-allotment, stabilizing transactions, short-covering transactions and penalty bids in accordance with Regulation M under the Securities Exchange Act of 1934, as amended. Over-allotment involves sales in excess of the offering size, which create a short position. Stabilizing transactions permit bids to purchase the underlying security so long as the stabilizing bids do not exceed a specified maximum price. Syndicate-covering or other short-covering transactions involve purchases of the securities, either through exercise of the over-allotment option or in the open market after the distribution is completed, to cover short positions. Penalty bids permit the underwriters to reclaim a selling concession from a dealer when the securities originally sold by the dealer are purchased in a stabilizing or covering transaction to cover short positions. Those activities may cause the price of the securities to be higher than it would otherwise be. If commenced, the underwriters may discontinue any of the activities at any time.
To comply with the securities laws of certain states, if applicable, the securities offered by this prospectus will be offered and sold in those states only through registered or licensed brokers or dealers.
In compliance with guidelines of the Financial Industry Regulatory Authority, or FINRA, the maximum consideration or discount to be received by any FINRA member or independent broker dealer may not exceed 8% of the aggregate amount of the securities offered pursuant to this prospectus and any applicable prospectus supplement.
The validity of the securities being offered hereby will be passed on by Stradling Yocca Carlson & Rauth, a Professional Corporation, Newport Beach, California.
The consolidated financial statements of VolitionRx Limited as of December 31, 2014 and 2013 and for each of the years in the two-year period ended December 31, 2014 have been incorporated by reference herein and in the registration statement in reliance upon the reports of Sadler, Gibb and Associates, LLC, our independent registered public accountant, incorporated by reference herein, and upon the authority of said firm as experts in accounting and auditing. The report of Sadler, Gibb and Associates, LLC dated March 18, 2015 notes that our losses and negative cash flow from operations raise substantial doubt that we will be able to continue as a going concern without further financing.
INCORPORATION OF CERTAIN INFORMATION BY REFERENCE
The SEC allows us to “incorporate” into this prospectus information that we file with the SEC in other documents. This means that we can disclose important information to you by referring to other documents that contain that information. Any information that we incorporate by reference into this prospectus is considered part of this prospectus.
Information contained in this prospectus and information that we file with the SEC in the future and incorporate by reference in this prospectus automatically modifies and supersedes previously filed information, including information in previously filed documents or reports that have been incorporated by reference in this prospectus, to the extent the new information differs from or is inconsistent with the old information. Any statement so modified will be deemed to constitute a part of this prospectus only as so modified, and any statement so superseded will be deemed not to constitute a part of this prospectus.
6
We incorporate by reference, as of their respective dates of filing, the documents listed below that we have filed with the SEC and any additional documents that we may file in the future with the SEC pursuant to Sections 13(a), 13(c), 14 or 15(d) of the Exchange Act, including any documents filed after the date of the initial registration statement of which this prospectus is a part until the offering of the security covered by this prospectus has been completed, other than, in each case, documents or information deemed to have been “furnished” and not “filed” in accordance with SEC rules:
our Annual Report on Form 10-K for the year ended December 31, 2014, as filed with the SEC on March 18, 2015;
our Quarterly Reports on Form 10-Q for the fiscal quarter ended March 31, 2015, as filed with the SEC on May 12, 2015, and for the fiscal quarter ended June 30, 2015, as filed with the SEC on August 11, 2015;
our Current Reports on Form 8-K as filed with the SEC on each of January 2, 2015, February 6, 2015 and August 17, 2015;
the description of our common stock contained in our registration statement on Form 8-A as filed with the SEC on February 3, 2015, as updated or amended in any amendment or report filed for such purpose.
We hereby undertake to provide without charge to each person, including any beneficial owner, to whom this prospectus is delivered, upon written or oral request, a copy of any or all documents that are incorporated by reference into this prospectus, but not delivered with the prospectus, other than exhibits to such documents unless such exhibits are specifically incorporated by reference into the documents that this prospectus incorporates. To request such materials, please contact Mr. Rodney Rootsaert, our Corporate Secretary at c/o Corporate Secretary, VolitionRx Limited, 1 Scotts Road, #24-05 Shaw Centre, Singapore, 228208, by email at notice@volitionrx.com, or by facsimile at +32 8172 5651. These documents are also available free of charge through the Investors section on our website at http://www.volitionrx.com as soon as practicable after such materials have been electronically filed with, or furnished to, the SEC.
You should rely only on the information contained in this prospectus, in any accompanying prospectus supplement, or in any document incorporated by reference herein or therein. We have not authorized anyone to provide you with any different information. We take no any responsibility for, and can provide no assurance as to the reliability of, any other information that others may provide to you. The information contained in this prospectus, in any applicable prospectus supplement, and in the documents incorporated by reference herein or therein is accurate only as of the date such information is presented. Our business, financial condition, results of operations and prospects may have changed since that date.
WHERE YOU CAN FIND MORE INFORMATION
We have filed a registration statement on Form S-3 with the SEC relating to the common stock, the preferred stock and the warrants offered by this prospectus. This prospectus does not contain all of the information set forth in the registration statement and the exhibits and schedules thereto. We have omitted parts of the registration statement, as permitted by the rules and regulations of the SEC. Statements contained in this prospectus as to the contents of any contract or other document referred to are not necessarily complete and in each instance reference is made to the copy of such contract or other document filed as an exhibit to the registration statement, each such statement being qualified in all respects by such reference. For further information with respect to us and the common stock, the preferred stock and the warrants offered hereby, reference is made to such registration statement, exhibits and schedules.
We are subject to the information and periodic reporting requirements of the Exchange Act, and in accordance therewith file periodic reports, current reports, proxy statements and other information with the SEC. Such periodic reports, current reports, proxy statements, other information and a copy of the registration statement on Form S-3 may be inspected by anyone without charge and copies of these materials may be obtained upon the payment of the fees prescribed by the SEC, at the Public Reference Room maintained by the SEC at 100 F Street, N.E., Washington, D.C. 20549. The public may obtain information on the operation of the Public Reference Room by calling the SEC at 1-800-SEC-0330. The registration statement on Form S-3 and the periodic reports, current reports, proxy statements and other information filed by us are also available through the Internet web site maintained by the SEC at the following address: http://www.sec.gov.
7

VOLITIONRX LIMITED
Shares of Common Stock
PROSPECTUS SUPPLEMENT
Sole Book-Running Manager
Oppenheimer & Co.
Co-Manager
National Securities Corporation
March , 2018