10-K: Annual report pursuant to Section 13 and 15(d)
Published on March 1, 2018
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 10-K
[X] ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934
For the Fiscal Year Ended December 31, 2017
[ ] TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE EXCHANGE ACT OF 1934
For the Transition Period from __________ to __________
Commission File Number: 001-36833
VOLITIONRX LIMITED
(Exact name of registrant as specified in its charter)
|
Delaware |
|
91-1949078 |
|
(State or other jurisdiction |
|
(I.R.S. Employer |
|
of incorporation or organization) |
|
Identification No.) |
|
|
#24-05 Shaw Centre Singapore 228208 (Address of principal executive offices)
|
|
|
|
Telephone: +1 (646) 650-1351 |
|
|
|
(Registrant’s telephone number, including area code) |
|
Securities registered pursuant to Section 12(b) of the Act:
|
Title of Each Class: |
Name of Each Exchange on Which Registered: |
|
Common Stock, par value $0.001 per share |
NYSE American, LLC |
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes [ ] No [X]
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes [ ] No [X]
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days. Yes [X] No [ ]
Indicate by check mark whether the registrant has submitted electronically and posted on its corporate Web site, if any, every Interactive Data File required to be submitted and posted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit and post such files). Yes [X] No [ ]
Indicate by check mark if disclosure of delinquent filers pursuant to Item 405 of Regulation S-K (§ 229.405 of this chapter) is not contained herein, and will not be contained, to the best of registrant’s knowledge, in definitive proxy or information statements incorporated by reference in Part III of this Form 10-K or any amendment to this Form 10-K. [X]
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act. (Check one):
|
Large accelerated filer |
[ ] |
Accelerated filer |
[ ] |
|
Non-accelerated filer |
[ ] (Do not check if a smaller reporting company) |
Smaller reporting company |
[X] |
|
Emerging growth company |
[ ] |
|
|
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. [ ]
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes [ ] No [X]
As of June 30, 2017, the last trading day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the voting common stock held by non-affiliates of the registrant was $72,868,822 (based upon the $3.54 per share closing price for the registrant’s common stock as reported by the NYSE American on such date). This calculation does not reflect a determination that persons deemed to be affiliates for this purpose are affiliates for any other purpose.
As of February 27, 2018, there were approximately 26,530,793 shares of the registrant’s common stock, $0.001 par value per share, outstanding.
Documents incorporated by reference: None
Table of Contents
|
|
|
Page |
|
PART I |
|
|
|
|
|
|
|
Item 1 |
BUSINESS |
2 |
|
Item 1A |
RISK FACTORS |
9 |
|
Item 1B |
UNRESOLVED STAFF COMMENTS |
19 |
|
Item 2 |
PROPERTIES |
19 |
|
Item 3 |
LEGAL PROCEEDINGS |
19 |
|
Item 4 |
MINE SAFETY DISCLOSURES |
19 |
|
|
|
|
|
PART II |
|
|
|
|
|
|
|
Item 5 |
MARKET FOR REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
20 |
|
Item 6 |
SELECTED FINANCIAL DATA |
20 |
|
Item 7 |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
21 |
|
Item 7A |
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
24 |
|
Item 8 |
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
F-1 |
|
Item 9 |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
25 |
|
Item 9A |
CONTROLS AND PROCEDURES |
25 |
|
Item 9B |
OTHER INFORMATION |
26 |
|
|
|
|
|
PART III |
|
|
|
|
|
|
|
Item 10 |
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
27 |
|
Item 11 |
EXECUTIVE COMPENSATION |
34 |
|
Item 12 |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS |
41 |
|
Item 13 |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE |
43 |
|
Item 14 |
PRINCIPAL ACCOUNTANT FEES AND SERVICES |
44 |
|
|
|
|
|
PART IV |
|
|
|
|
|
|
|
Item 15 |
EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
45 |
|
Item 16 |
FORM 10-K SUMMARY |
49 |
|
|
|
|
|
|
|
|
|
SIGNATURES |
|
50 |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K for the fiscal year ended December 31, 2017, which we refer to as this report, contains “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended, or the Securities Act, and Section 21E of the Securities Exchange Act of 1934, as amended, or the Exchange Act, which statements are subject to considerable risks and uncertainties. These forward-looking statements are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical fact included in this report or incorporated by reference into this report are forward-looking statements. Throughout this report, we have attempted to identify forward-looking statements by using words such as “may,” “believe,” “will,” “could,” “project,” “anticipate,” “expect,” “estimate,” “should,” “continue,” “potential,” “plans,” “forecasts,” “goal,” “aim,” “seek,” “intend,” other forms of these words or similar words or expressions or the negative thereof (although not all forward-looking statements contain these words). In particular, forward looking statements contained in this report relate to, among other things, any predictions of earnings, revenues, expenses or other financial items; plans or expectations with respect to our development activities or business strategy, including commercialization and market acceptance; statements concerning industry trends and industry size; statements regarding anticipated demand for our products and market opportunity, or the products of our competitors; statements relating to manufacturing forecasts, and the potential impact of our relationship with contract manufacturers and original equipment manufacturers on our business; assumptions regarding the future cost and potential benefits of our research and development efforts; the effect of critical accounting policies; forecasts of our liquidity position or available cash resources; statements relating to the impact of pending litigation; and statements relating to the assumptions underlying any of the foregoing.
We have based our forward-looking statements on our current expectations and projections about trends affecting our business and industry and other future events. Although we do not make forward-looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy. Forward-looking statements are subject to substantial risks and uncertainties that could cause our future business, financial condition, results of operations or performance, to differ materially from our historical results or those expressed or implied in any forward-looking statement contained in this report. We discuss these risks and uncertainties in greater detail in the section entitled “Risk Factors” in Part I, Item 1A of this report, and the other documents that we have filed with the Securities and Exchange Commission, or the SEC.
In addition, actual results may differ as a result of additional risks and uncertainties of which we are currently unaware or which we do not currently view as material to our business. For these reasons, readers are cautioned not to place undue reliance on any forward-looking statements.
You should read this report in its entirety, the documents that we file as exhibits to this report and the documents that we incorporate by reference into this report, with the understanding that our future results may be materially different from what we currently expect. The forward-looking statements we make speak only as of the date on which they are made. We expressly disclaim any intent or obligation to update any forward-looking statements after the date hereof to conform such statements to actual results or to changes in our opinions or expectations. If we do update or correct any forward-looking statements, readers should not conclude that we will make additional updates or corrections.
Use of Terms
Except as otherwise indicated by the context, references in this report to “Company,” “VolitionRX,” “Volition,” “we,” “us”, and “our” are references to VolitionRX Limited and its wholly-owned subsidiaries, Singapore Volition Pte. Limited, Belgian Volition SPRL, Hypergenomics Pte. Limited, Volition Diagnostics UK Limited and Volition America, Inc. Additionally, unless otherwise specified, all references to “$” refer to the legal currency of the United States of America.
Nucleosomics®, Nu.QTM and Hypergenomics® and their respective logos are trademarks and/or service marks of VolitionRX and its subsidiaries. All other trademarks, service marks and trade names referred to in this report are the property of their respective owners.
1
ITEM 1.BUSINESS
Overview
VolitionRX is a multi-national life sciences company developing simple, easy to use, cost effective blood tests to help diagnose a range of cancers. We hope that through earlier diagnosis we can help save and improve the quality of many people’s lives throughout the world.
Our Solution/ Science
Our tests are based on the science of Nucleosomics®, which is the practice of identifying and measuring nucleosomes in the bloodstream or other bodily fluid - an indication that disease is present.
The principle behind what we are doing relies on bringing together two main lines of research and is, in concept, very simple: the chromosomes of cancer cells differ from those of healthy cells – both in terms of DNA sequence (due to genetic cancer mutations) and in protein structure - due to epigenetic changes. There are chromosome fragments from dead cancer cells circulating in the blood as nucleosomes. Each such circulating nucleosome contains a small (approx. 140bp) fragment of tumor DNA.
Our Nucleosomics technology exploits the different compositions of circulating nucleosome structures present in the serum of cancer patients to detect and identify cancer diseases.
We have developed a novel suite of blood assays for epigenetically altered circulating nucleosomes as biomarkers in cancer. Nu.QTM products are simple, low-cost, ELISA platform tests and can incorporate other off-patent, low-cost ELISA tests in our panels (e.g. CEA, PSA, CA125) for higher accuracy.
Many companies and medical schools are developing circulating tumor DNA, or ctDNA, tests based on sequencing the DNA attached to these nucleosomes.
Our diagnostic target in the blood is the same tumor chromosome fragment, but our approach is to test for chromosome protein and nucleic acid changes in intact chromosome fragments by ELISA, rather than chemically extracting, amplifying, and sequencing the ctDNA and discarding the rest of the nucleosome. ELISA is possible because the targets of our tests occur globally across all nucleosomes within a tumor cell, whereas individual ctDNA changes must be identified within the three billion base-pair genomes. This means that the targets of our tests are exponentially more prevalent in circulating blood, and detectable using simple laboratory methods.
How is Nu.Q different from ctDNA?
When a cancer cell dies the nuclear components are metabolized into 20 million individual DNA-Nu complexes and released into circulation. A cancer mutation will occur in one of the DNA-Nu complexes.
ctDNA sequencing methods (in development) must target that one-in-20 million DNA-Nu complexes.
Nu.QTM targets all 20 million circulating DNA-Nu complexes because nucleosome modifications occur globally.
Nu.Q is a simple low-cost ELISA and can incorporate other ELISA tests in our panels.
Using our Nucleosomics technology, we have developed 39 epigenetic Nu.Q assays, which are designed to detect the level and structure of nucleosomes in blood. Epigenetics is the science of how genes are switched “on” or “off” in the body’s cells. A major factor controlling the switching “on” and “off” is the structuring of DNA. The DNA in human cells is packaged as protein complexes in a “beads on a string” structure. Each individual protein/DNA “bead” is called a nucleosome. These nucleosomes then form additional structures with increasingly dense packing, culminating in chromosomes containing hundreds of thousands of nucleosomes as depicted in Figure 1 below.
2
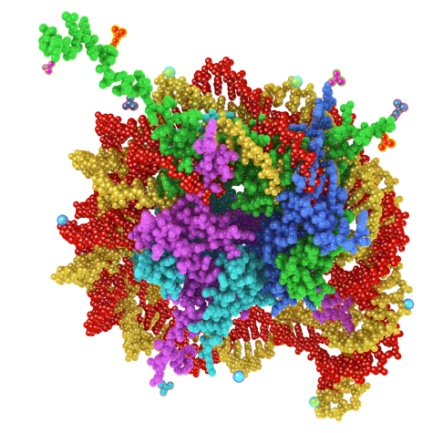
Figure 1 – A nucleosome
Cancer is characterized by uncontrolled and often rapid cell growth which exceeds the corresponding rate of cell death. When cells die, the DNA fragments into individual nucleosomes which are released into the blood as illustrated in Figure 2 below. The cell debris in the bloodstream is eventually recycled back into the body. When a cancer is present, the number of dying cells can overwhelm the recycling process, leaving the excess fragments, including the nucleosomes, in the blood. Importantly, the structure of nucleosomes is not uniform but subject to immense variety, and nucleosomes in cancer cells have differences in structure from those in healthy cells.
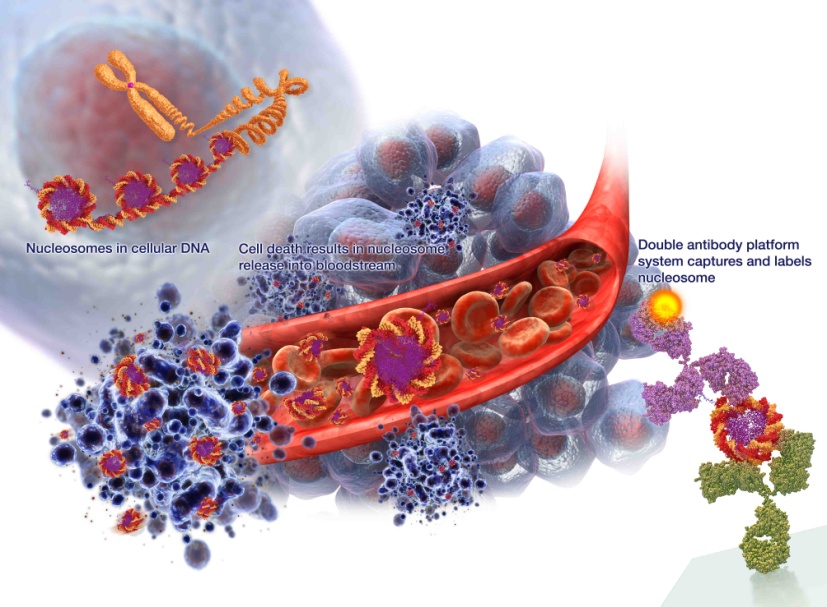
Figure 2 – Release of nucleosomes into blood
Blood nucleosome levels can be raised in conditions other than cancer including in auto-immune disease, inflammatory disease, endometriosis, sepsis, and in the immediate aftermath of major trauma (for example following a heart attack, surgery or car accident). Our primary focus is on cancer diagnosis, but we also intend to pursue diagnostic opportunities in other disease areas.
Research and Development
We are developing blood-based tests for the most prevalent cancers, beginning with colorectal cancer, or CRC. Following CRC, we anticipate focusing on lung cancer, prostate and pancreatic cancer, using our Nucleosomics biomarker discovery platform. Our development pipeline includes assays to be used for symptomatic patients or asymptomatic (screening) populations. The platform employs a range of simple Nu.Q immunoassays on an industry standard ELISA format, which allows rapid quantification of epigenetic changes in biofluids (whole blood, plasma, serum, sputum, urine etc.) compared to other approaches such as bisulfite conversion and polymerase chain reaction, or PCR.
We have developed 39 blood-based Nu.Q immunoassays to date to detect specific biomarkers that can be used individually or in combination to generate a profile which forms the basis of a product for a particular cancer or disease.
Research and development expenses increased to $8.9 million for the year ended December 31, 2017 from $7.9 million for the year ended December 31, 2016. This increase in overall research and development expenditures was primarily related to additional costs of $0.5 million due our participation in the 13,500 patient trial with the National Cancer Institute’s Early Detection Research Network in collaboration with the University of Michigan.
3
Clinical Studies
Listed below are the major studies we have underway in colorectal cancer, pancreatic cancer and a study involving 27 cancers. Further studies in colorectal cancer in Taiwan are planned to commence in the second half of 2018.
|
Institution |
Condition |
Sample Collection |
Cohort |
Timing |
|
Early Detection Research Network of the U.S. National Cancer Institute (EDRN)
|
Colorectal Cancer |
9,000 Prospective, 4,600 Retrospective |
13,500 + Screening Population |
Collection Ongoing to 2020 |
|
Hvidovre Hospital, University of Copenhagen
|
Colorectal Cancer |
Prospective |
14,000 Screening Population |
Collection completed. Results expected in 2018 |
|
Hvidovre Hospital, University of Copenhagen |
Colorectal Cancer |
Prospective, Longitudinal |
30,000 Screening Population (to provide 3 samples each)
|
Ongoing to 2022 |
|
Hvidovre Hospital, University of Copenhagen
|
Colorectal Cancer |
Retrospective |
4,800 Symptomatic Patients |
Collection completed. Final Results expected in 2018 |
|
University of Bonn |
27 most prevalent cancers
|
Prospective |
4,500 Subjects |
Collection completed. Results expected in 2018 |
|
German Cancer Research Center (DKFZ) |
Pancreatic Cancer |
Retrospective |
750 Subjects |
Collection completed. Results expected in 2018/19 |
Commercialization Strategy
We are transitioning from a purely clinical stage company to a commercial company. We will continue to research and develop additional assays and products across a range of cancers as we continue to develop our commercial operations. We plan to develop multiple products across the whole range of cancers falling into the categories listed below:
|
Frontline General Population Screening Tests for asymptomatic subjects for the most prevalent cancers
For example, lung, colorectal, gastric and breast cancers. |
|
High Risk Screening “Triage” Tests to work in conjunction with existing tests to improve sensitivity and/or specificity
For example, with FIT for colorectal cancer. |
|
Frontline Diagnostic / Adjunct Diagnostic Tests to aid the diagnosis of disease in symptomatic patients and/or high- risk patients
For example, with low dose CT scans for lung cancer or Type II diabetes patients for pancreatic cancer. |
|
Disease Monitoring Tests to help monitor and/or identify the recurrence of a disease
For example, prostate cancer. |
We believe that given the global prevalence of cancer and the low cost, accessible routine nature of our tests, Nu.Q will eventually be used throughout the world. Our launch sequence is determined to a large extent by regulatory hurdles - consequently, we aim to launch first in Europe, then in Asia, and subsequently in the United States. We plan to work with partners and/or distributors to commercialize Nu.Q worldwide.
If we do not have enough funds to fully implement our business plan, we will be forced to scale back our plan of operations and our business activities, increase our anticipated timeframes to complete each milestone or seek additional funding. In the event that additional financing is delayed, we will prioritize the maintenance of our research and development personnel and facilities, primarily in Belgium.
4
The Market Opportunity
Cancer is one of the leading causes of death worldwide, accounting for around 8.2 million annual deaths globally. There are over 14 million new cases of cancer diagnosed each year and given the aging population this is expected to grow rapidly to over 21.5 million new cases annually by 2030. By way of example as of today, in the United States there are more than three new cases of cancer diagnosed and one person dies of a cancer-related death every minute.
Statistically, the chances of surviving cancer are greatly improved by early detection and treatment. However, there are currently very few blood tests for diagnosis of cancer in common clinical use. The only blood test commonly used for screening any cancer is the Prostate-Specific Antigen, or PSA, test for prostate cancer. We consider the PSA test to have relatively poor diagnostic accuracy (detecting approximately 70% of prostate cancers and misdiagnoses of about 30% of healthy men as positive for cancer) but it is widely used because it is the best product currently available. The PSA test is intended to be used to monitor patients after definitive diagnosis or treatment. The American Cancer Society recommends that prostate cancer screening should not occur without an informed decision-making process regarding risks. In 2012, the U.S. Preventative Services Task Force recommended against PSA- based screening for healthy men because of a “moderate or high probability” that the service has no benefit or that the harms outweigh the benefits. There are few currently commonly used approved blood tests for screening for cancer.
Our initial and current focus is colorectal cancer, or CRC, which is the third leading cause of cancer deaths worldwide accounting for almost 700,000 lives lost annually. In the United States, CRC is the second leading cause of cancer deaths and the leading cause of cancer deaths in the United States among non-smokers. Each year in the United States there are over 140,000 new cases of CRC and approximately 51,000 deaths from CRC.
The Journal of the National Cancer Institute claims that, while CRC is the most preventable cancer, it remains the least prevented form of cancer. Current methods of CRC diagnosis are either invasive, not cost-effective, have low acceptance or cannot provide accurate results. The inadequacy of existing diagnostic products means that most cancers are only diagnosed once the patient experiences symptoms and the cancer is well established. By this stage, it will often have spread beyond the primary tumor (metastatic cancers), making it substantially more difficult to treat. For example, while the overall 5-year survival rate for cancer patients is 65%, the survival rate differs significantly depending upon the stage of diagnosis. Only 14% of patients diagnosed at Stage IV survive 5 or more years whereas 90% of those diagnosed at Stage I survive five or more years; once more underlining the importance of early detection. We believe that early, non-invasive, accurate cancer diagnosis remains a significant unmet medical need and a significant commercial opportunity. For these reasons, cancer diagnostics is an active field of research and development both academically and commercially.
The global in vitro diagnostic medical device, or IVD, market is forecasted to reach $65 billion in 2018; driven by the increasing health care demands of an aging population. In the United States, the IVD market is primarily comprised of:
Immunochemistry of tissue samples (expected to grow 6.8% per annum from 2011-2018, with an expected value of $25.5 billion by 2018). These tests are mostly used to confirm cancer diagnosis post-surgery and to determine cancer sub-type;
Immunoassay (chemical tests used to detect a substance in blood or body fluid), is expected to be the second largest market with a value of more than $19.1 billion by 2018. These tests are mostly used to monitor for disease progress and relapse. This market segment includes our future Nucleosomics products, which will be blood-based immunoassay tests for modified nucleosomes for the diagnosis of cancer.
We anticipate that because of their ease of use and cost efficiency, our tests have the potential to become the first method of choice for cancer diagnostics, allowing detection of a range of cancers at an earlier stage than typically occurs currently, and testing of individuals who, for reasons such as time, cost or aversion to current methods, are not currently being tested. We believe our frontline blood test for CRC has the potential to have significantly higher compliance from patients compared to current fecal immunochemical tests, or FIT, and colonoscopies which are invasive and/or unpleasant. Our frontline blood test, currently in development, could be of significant benefit to screen relevant individuals (aged 50 to 74 years old).
5
Competition
We believe that Epigenomics AG, or Epigenomics, is our main competitor in the blood-based diagnostic market. Epigenomics’ methylated DNA-based PCR test in colon cancer (Epi proColon®) is available in the United States, Europe, China and select other countries and its lung cancer test (Epi proLung®) has been CE-Marked in Europe. CellMax Life is another cancer diagnostics company offering non-invasive tests for early cancer detection and management, however, currently, its tests have limited clinical data and are available only in Taiwan. In colon cancer, our main target market, we also face potential competition from alternative procedures including flexible sigmoidoscopy, colonoscopy and virtual colonoscopy as well as traditional tests such as the stool guaiac and FIT. Exact Sciences Corporation has FDA and reimbursement approval for its stool-based DNA screening test, Cologuard®. We anticipate facing competition primarily from healthcare, pharmaceutical and diagnostic companies such as Epigenomics, Exact Sciences Corporation, Abbott Laboratories Inc., Cepheid Inc., Philips, GE Healthcare, Siemens, Gen-Probe Incorporated, MDxHealth SA, and Roche Diagnostics. There may also be other companies developing products competitive with ours of which we are unaware.
We hope that our future products will have a competitive edge compared to those offered by competitors on the basis that our tests are being developed to be accurate, cost-effective and attractive from a government reimbursement perspective, easy to use, non-invasive, technologically advanced, and compatible with ELISA systems, based on strong intellectual property and to be used for mass screenings.
Many of our anticipated competitors have substantially greater financial, technical, and other resources and larger, more established marketing, sales and distribution systems than we have. Many of our competitors also offer broad product lines outside of the diagnostic testing market and have brand recognition. Moreover, our competitors may make rapid technological developments that may result in our intended technologies and products becoming obsolete before we are able to enter the market, recover the expenses incurred to develop them or generate significant revenue. Our success will depend, in part, on our ability to develop our intended products in a timely manner, keep our future products current with advancing technologies, achieve market acceptance of our future products, gain name recognition and a positive reputation in the healthcare industry, and establish successful marketing, sales and distribution efforts.
Government Regulations
The health care industry, and thus our business, is subject to extensive federal, state, local and foreign regulation. Some of the pertinent laws have not been definitively interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of subjective interpretations. In addition, these laws and their interpretations are subject to change.
Both United States federal and state governmental agencies continue to subject the health care industry to intense regulatory scrutiny, including heightened civil and criminal enforcement efforts. As indicated by work plans and reports issued by these agencies, the federal government will continue to scrutinize, among other things, the marketing, labeling, promotion, manufacturing and export of diagnostic health care products. Our diagnostic products fall within the IVD medical device category and are subject to FDA clearance or approval in the United States.
The federal government also has increased funding in recent years to fight health care fraud, and various agencies, such as the United States Department of Justice, the Office of Inspector General of the Department of Health and Human Services, or OIG, and state Medicaid fraud control units, are coordinating their enforcement efforts.
In Europe, medical devices are regulated by self-certification through the CE Mark system. Under the system, developers and manufacturers must operate a Quality System and validate medical devices in a limited clinical trial to demonstrate the manufacturer has met analytical and clinical performance criteria. We have implemented an International Organization for Standardization standard - ISO 13485 - quality management system for the design and manufacture of medical devices. ISO 13485 addresses managerial awareness of regulatory requirements, control systems, inspection and traceability, device design, risk and performance criteria as well as verification for corrective and preventative measures for device failure. Medical device companies such as ours are subject to pre-market compliance assessments from Notified Bodies, a certification organization which the national authority (the competent authority) of a European Union member state designates to carry out one or more of the conformity assessment procedures. ISO 13485 certification establishes conformity to specific European Union directives related to medical devices and allows CE Marking and sale of the device.
The new European In Vitro Diagnostic Regulation (IVDR - 2017/746), or the IVDR, became effective as of May 25, 2017, marking the start of a transition period for manufacturers selling IVD devices into Europe. The IVDR, which replaces IVD Directive (98/79/EC), or the Directive, has a transition period of five years, after which the IVDR will apply in full, and no new applications pursuant to the Directive will be accepted. Manufacturers have the duration of the five-year transition period to update their technical documentation and processes to meet the new, more stringent EU regulatory requirements. We believe that the most challenging areas under the IVDR will be regarding the classification of products, which will bring almost all IVDs under the direct control of Notified Bodies, and the performance evaluation of IVDs, which will not only include the classic clinical performance and analytical performance but also scientific validity, the role and responsibilities of the economic actors of the supply chain, the traceability and the transparency of the devices with, in particular, the introduction of the UDI-system and an expanded EUDAMED database.
6
Notified Bodies can begin auditing to the IVDR once they have been designated as a Notified Body under the IVDR by their Competent Authority. For now, we expect the first Notified Bodies to be notified according the IVDR by the end of 2019 and we anticipate that TÜV SÜD will be one of these. In practice, it will not be possible to CE mark a product according to the IVDR beforehand. For Class C devices (Belgian Volition’s devices should be Class C), the conformity assessment procedure will be a combination of the Quality Management System audits and Technical Documentation assessments. The assumed assessment time needed for a Technical Documentation assessment of a Class C device is expected to last from about 2 months to 6 months. Belgian Volition has already begun discussions with the TÜV SÜD in order to ensure compliance with the IVDR as soon as possible.
We will also be required to comply with numerous other federal, state, and local laws relating to matters such as safe working conditions, industrial safety, and labor laws. We may incur significant costs to comply with such laws and regulations in the future, and lack of compliance could have material adverse effects on our operations.
We believe that we have structured our business operations to comply with applicable legal requirements. However, it is possible that governmental entities or other third parties could interpret these laws differently and assert otherwise, which could have a material adverse impact on our business.
Regulatory Approach
Commercialization of our future products in the clinical IVD market (e.g. for patient diagnosis in hospitals, clinics, etc.) requires government approval (CE Marking in Europe, FDA approval in the United States, and Chinese Food and Drug Administration, or CFDA, approval in China).
In the United States, we anticipate that our tests will have to be cleared through the FDA’s premarket notification or 510(k), process or its premarket approval, or PMA, process. The determination of whether a 510(k) or a PMA is necessary will depend in part on the proposed indications for use and the FDA’s assessment of the risk associated with the use of the IVD for a particular indication. A similar system operates in China through the CFDA. In the European Union, our tests can be marketed after a declaration and marking that the test conforms to the essential requirements of the relevant European health, safety and environmental protection legislation, or CE Marking. The CE Mark is also recognized in certain Asian territories, including India, for the private payer market.
We obtained our first CE Mark in September 2015, for a single biomarker for CRC, and two further CE Marks in April 2016, for two biomarkers for CRC and pancreatic cancer. In December 2016, we achieved a CE Mark for the Nu.Q Colorectal Cancer Screening Triage Test.
We are currently working on different products such as a frontline screening test and a symptomatic test for CRC. We expect that we will be required to perform additional clinical trials in the United States to obtain FDA clearance or approval for these CRC tests. We are committed to obtaining FDA clearance or approval to allow patient access to our tests in the United States as soon as practicable.
We also expect that we will be required to do trials in China to achieve CFDA approval for our various tests, provided we can ensure adequate protection of our intellectual property in China. Local validation studies will be required to support sales of our CE-Marked CRC test in many Asian markets for the private payer market.
We have 17 patent families related to our diagnostic tests, with five patents granted in the United States and four patents granted in the European Union. Additionally, we have 12 patent applications in the name of our subsidiaries pending in the United States and the 13 patent applications in the European Union.
We intend to continue our development of the Nucleosomics technologies and will continue to apply for patents for future product developments. Our strategy is to protect the technologies and gain market exclusivity with patents in Europe and the United States and in other strategic countries. The patents on the technologies underlying our products should provide broad coverage for each product, including protection through at least 2031 for products developed using the Nu.Q-X, Nu.Q-V and Nu.Q-A technologies.
7
Employees
As of December 31, 2017, we (including our subsidiaries) had 37 full-time equivalents compared to 28 as of December 31, 2016.
Corporate History
The Company was incorporated on September 24, 1998 in the State of Delaware under the name “Standard Capital Corporation”. On September 22, 2011, the Company filed a Certificate for Renewal and Revival of Charter with the Secretary of State of Delaware. Pursuant to Section 312 of Delaware General Corporation Law, the Company was revived under the new name of “VolitionRX Limited”. The Company acquired its wholly-owned operating subsidiary, Singapore Volition Pte. Limited, a Singapore registered company, or Singapore Volition, on October 6, 2011. Singapore Volition has two subsidiaries, Belgian Volition SPRL, a Belgium private limited liability company, or Belgian Volition, which it acquired on September 22, 2010, and Hypergenomics Pte. Limited, a Singapore registered company, or Hypergenomics, which it formed on March 7, 2011. Belgian Volition has two subsidiaries, Volition Diagnostics UK Limited, which it formed on November 13, 2015, and Volition America, Inc., which it formed on February 3, 2017.
Our principal executive office is located at 1 Scotts Road, #24-05 Shaw Centre, Singapore 228208. Our telephone number is +1 (646) 650-1351. Our website is located at www.volitionrx.com. The information that can be accessed through our website is not incorporated by reference into this report and should not be considered to be a part hereof.
Financial Information
See our consolidated financial statements included in this Form 10-K and accompanying notes to the consolidated financial statements.
WHERE YOU CAN GET ADDITIONAL INFORMATION
We file Annual Reports on Form 10-K, Quarterly Reports on Form 10-Q, and Current Reports on Form 8-K pursuant to Section 13(a) or 15(d) of the Exchange Act electronically with the SEC. You may read and copy our reports or other filings made with the SEC at the SEC’s Public Reference Room, located at 100 F Street, N.E., Washington, DC 20549 on official business days during the hours of 10:00 a.m. and 3:00 p.m. You can obtain information on the operations of the Public Reference Room by calling the SEC at 1-800-SEC-0330. You can also access these reports and other filings electronically on the SEC’s web site, www.sec.gov.
8
ITEM 1A.RISK FACTORS
An investment in our securities involves certain risks, including those set forth below and elsewhere in this report. In addition to the risks set forth below and elsewhere in this report, other risks and uncertainties may exist that could adversely affect our business and financial condition. If any of the following risks actually materialize, our business, financial conditions and/or operations could suffer. In such event, the value of our common stock could decline, and you could lose all or a substantial portion of your investment. You should carefully consider the risks described below as well as other information and data included in this report.
Risks Associated with our Company
We have not generated any significant revenue since our inception and we may never achieve profitability.
We are a clinical stage company and have incurred losses since our formation. As of December 31, 2017, we have an accumulated total deficit of approximately $55.7 million. As we continue the discovery and development of our future diagnostic products, our expenses are expected to increase significantly. Even as we begin to market and sell our intended products, we expect our losses to continue as a result of ongoing research and development expenses, as well as increased manufacturing, sales and marketing expenses. These losses, among other things, have had and will continue to have an adverse effect on our working capital, total assets and stockholders’ equity. Because of the numerous risks and uncertainties associated with our product development and commercialization efforts, we are unable to predict when we will become profitable, and we may never become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. If we are unable to achieve and then maintain profitability, our business, financial condition and results of operations will be negatively affected and the market value of our common stock will decline.
We may need to raise additional capital in the future. If we are unable to secure adequate funds on terms acceptable to us, we may be unable to execute our plan of operations.
We will require additional capital to fully fund our current strategic plan, which includes successfully commercializing our Nu.Q colorectal cancer pipeline and developing future products. If we incur delays in commencing commercialization of our Nu.Q colorectal cancer pipeline or other future products or in achieving significant product revenue, or if we encounter other unforeseen adverse business developments, we may exhaust our capital resources prior to the commencement of commercialization.
We cannot be certain that additional capital will be available when needed or that our actual cash requirements will not be greater than anticipated. Financing opportunities may not be available to us, or if available, may not be available on favorable terms. The availability of financing opportunities will depend on various factors, such as market conditions and our financial condition and outlook. In addition, if we raise additional funds through the issuance of equity or convertible debt securities, the percentage ownership of our stockholders could be significantly diluted, and these newly-issued securities may have rights, preferences or privileges senior to those of existing stockholders. If we obtain debt financing, a substantial portion of our operating cash flow may be dedicated to the payment of principal and interest on such indebtedness, and the terms of the debt securities issued could impose significant restrictions on our operations. If we are unable to obtain financing on terms favorable to us, we may be unable to execute our plan of operations and we may be required to cease or reduce development or commercialization of any future products, sell some or all of our technology or assets or merge with another entity.
It is difficult to forecast our future performance, which may cause our financial results to fluctuate unpredictably.
Our limited operating history and the rapid evolution of the market for diagnostic products make it difficult for us to predict our future performance. A number of factors, many of which are outside of our control, may contribute to fluctuations in our financial results, such as:
our ability to develop or procure antibodies for clinical use in our future products;
our ability to translate preliminary clinical results to larger prospective symptomatic and screening populations;
the demand for our intended products;
our ability to obtain any necessary financing;
our ability to market and sell our future products;
market acceptance of our future products and technology;
performance of any future strategic business partners;
our ability to obtain regulatory clearances or approvals;
our success in collecting payments from third-party payors and customers;
changes in technology that may render our future products uncompetitive or obsolete;
competition with other cancer diagnostics companies; and
adverse changes in the healthcare industry.
9
Our future success depends on our ability to retain our officers and directors, scientists, and other key employees and to attract, retain and motivate qualified personnel.
Our success depends on our ability to attract, retain and motivate highly qualified management and scientific personnel. In particular, we are highly dependent on Cameron Reynolds, our President and Chief Executive Officer, our other officers and directors, scientists and key employees. The loss of any of these persons or their expertise would be difficult to replace and could have a material adverse effect on our ability to achieve our business goals. In addition, the loss of the services of any one of these persons may impede the achievement of our research, development and commercialization objectives by diverting management’s attention to the identification of suitable replacements, if any. There can be no assurance that we will be successful in hiring or retaining qualified personnel and our failure to do so could have a material adverse effect on our business, financial condition and results of operations.
Recruiting and retaining qualified scientific personnel and, in the future, sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among pharmaceutical, biotechnology and diagnostic companies for similar personnel. We also experience competition for the hiring of scientific personnel from universities and research institutions. We do not maintain “key person” insurance on any of our employees. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research, development and commercialization strategies. Our consultants and advisors, however, may have other commitments or employment that may limit their availability to us.
We expect to expand our product development, research and sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We are focused on developing our pipeline for future products. Our efforts will result in significant growth in the number of our consultants, advisors, and employees and the scope of our operations. In order to manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities, and continue to recruit and train additional qualified personnel. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plan or disrupt our operations.
We have limited experience with direct sales and marketing and any failure to build and manage a direct sales and marketing team effectively, or to successfully engage third party providers for such services, could have a material adverse effect on our business.
Our products will require several dynamic and evolving sales models tailored to different worldwide markets, users and products. In 2015, we decided to focus our sales strategy on the clinical IVD market with the CE Marking of our first product in Europe. Following CE Marking of our first product in Europe we intend to enter the European markets and, following the completion of any necessary regulatory clearances, certain Asian markets. Even when we have received a CE Mark, we must still seek regulatory clearance in other jurisdictions. A failure to obtain these regulatory clearances in other jurisdictions could negatively affect our business. Pending completion of our review of the regulatory environment in the United States, including the effect of recent pronouncements regarding LDTs by the FDA, we may decide to enter the United States market through a CLIA certified laboratory in the United States. We intend to progressively grow to large volumes of tests sold to centralized laboratories and eventually reach the mass diagnostics testing market. The exact nature of the ideal sales strategy will evolve as we continue to develop our intended products and seek entry into the IVD markets. We have limited experience with direct sales and marketing and we currently intend to engage a network of distributors to help commercialize our products worldwide. Any failure to build and manage a direct sales and marketing team effectively, or to successfully engage third party providers for such services, could have a material adverse effect on our business.
There are significant risks involved in building and managing our sales and marketing organization, as well as identifying and negotiating deals with the right sales and distribution partners, including risks related to our ability to:
identify appropriate partners;
negotiate beneficial partnership and distribution agreements;
hire qualified individuals as needed;
generate sufficient leads within our targeted market for our sales force;
provide adequate training for effective sales and marketing;
protect intellectual property rights;
retain and motivate our direct sales and marketing professionals; and
effectively oversee geographically dispersed sales and marketing teams.
10
Our failure to adequately address these risks could have a material adverse effect on our ability to increase sales and use of our future products, which would cause our revenues to be lower than expected and harm our results of operations.
Our Second Amended and Restated Certificate of Incorporation exculpates our officers and directors from certain liability to our Company and our stockholders.
Our Second Amended and Restated Certificate of Incorporation contains a provision limiting the liability of our officers and directors for their acts or failures to act, except for acts involving intentional misconduct, fraud or a knowing violation of law. This limitation on liability may reduce the likelihood of derivative litigation against our officers and directors and may discourage or deter our stockholders from suing our officers and directors based upon breaches of their duties to our Company.
We have identified material weaknesses in our internal control over financial reporting that have not yet been remediated, and the failure to address these material weaknesses, or the identification of any others, could impact the reliability of our financial reporting and harm investors’ views of us, which could adversely impact our stock price.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. As defined in Exchange Act Rule 13a-15(f), internal control over financial reporting is a process designed by, or under the supervision of, the principal executive and principal financial officer and effected by the board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and dispositions of assets;
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and/or directors; and
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements.
We have determined that we have material weaknesses in our internal control over financial reporting as of December 31, 2017. See Item 9A. Controls and Procedures of this report for a complete discussion of these material weaknesses in our internal control over financial reporting and remediation efforts. Although we are undertaking steps to address these material weaknesses, the existence of a material weakness is an indication that there is more than a remote likelihood that a material misstatement of our financial statements will not be prevented or detected in the current or any future period. There can be no assurance that we will be able to fully implement our plans and controls, as further described in Item 9A, to address these material weaknesses, or that the plans and controls, if implemented, will be successful in fully remediating these material weaknesses. In addition, we may in the future identify further material weaknesses in our internal control over financial reporting that we have not discovered to date. If we fail to successfully remediate the identified material weaknesses, or we identify further material weaknesses in our internal controls, the market’s confidence in our financial statements could decline and the market price of our common stock could be adversely impacted. Additionally, for so long as we remain as a smaller reporting company, under current rules our accounting firm will not be required to provide an opinion regarding our internal controls over financial reporting.
We have a “going concern” opinion from our auditors, indicating the possibility that we may not be able to continue to operate.
Our independent registered public accountants have expressed substantial doubt about our ability to continue as a going concern. This opinion could materially limit our ability to raise additional funds by issuing new debt or equity securities or otherwise. If we fail to raise sufficient capital when needed, we will not be able to complete our proposed business plan. As a result we may have to liquidate our business and investors may lose their investments. Our ability to continue as a going concern is dependent upon our ability to successfully accomplish our plan of operations described herein, obtain financing and eventually attain profitable operations. Investors should consider our independent registered public accountant’s comments when deciding whether to invest in the Company.
Our management has broad discretion over the use of our available cash and might not spend available cash in ways that increase the value of your investment.
As of December 31, 2017, we had $10.1 million in combined cash and marketable securities compared to $21.7 million as of December 31, 2016. Our management currently expects to deploy these resources primarily to expand our commercialization activities, to fund our product development efforts and for general corporate and working capital purposes. However, our management has broad discretion to pursue other objectives. You will be relying on the judgment of our management regarding the application and prioritization of our resources. Our management might not apply our cash in ways that increase or permit any return of your investment.
11
Risks Associated with our Business
Failure to successfully develop, manufacture, market, and sell our future products will have a material adverse effect on our business, financial condition, and results of operations.
We are in the process of developing a suite of diagnostic tests as well as additional products. The successful development and commercialization of our intended products is critical to our future success. Our ability to successfully develop, manufacture, market, and sell our future products is subject to a number of risks, many of which are outside our control. There can be no assurance that we will be able to develop and manufacture products in commercial quantities at acceptable costs, successfully market any products, or generate revenues from the sale of any products. Failure to achieve any of the foregoing would have a material adverse effect on our business, financial condition, and results of operations.
Our business is dependent on our ability to successfully develop and commercialize diagnostic products. If we fail to develop and commercialize diagnostic products, we may be unable to execute our plan of operations.
Our current business strategy focuses on discovering, developing and commercializing diagnostic products. The success of our business will depend on our ability to fully develop and commercialize the diagnostic products in our current development pipeline as well as continue the discovery and development of other diagnostics products. Currently, we are heavily dependent on our Nu.Q colorectal cancer pipeline. The commercial success of our Nu.Q colorectal cancer pipeline will impact our ability to generate revenues.
Prior to commercializing the Nu.Q Colorectal Cancer Screening Triage Test and other diagnostic products, we will be required to undertake time-consuming and costly development activities with uncertain outcomes, including conducting clinical studies and obtaining regulatory clearance or approval in the United States and in Europe. Delays in obtaining approvals and clearances could have material adverse effects on us and our ability to fully carry out our plan of operations. We have limited experience in taking products through these processes and there are considerable risks involved in these activities. The science and methods that we are employing are innovative and complex, and it is possible that our development programs will ultimately not yield products suitable for commercialization or government approval. Products that appear promising in early development may fail to be validated in subsequent studies, and even if we achieve positive results, we may still fail to obtain the necessary regulatory clearances or approvals. Few research and development projects result in commercial products, and perceived viability in early clinical studies often is not replicated in later studies. At any point, we may abandon development of a product, or we may be required to expend considerable resources obtaining additional clinical and nonclinical data, which would adversely impact the timing for generating potential revenue from those products. Further, our ability to develop and launch diagnostic tests is dependent on our receipt of substantial additional funding. If our discovery and development programs yield fewer commercial products than we expect, we may be unable to execute our business plan, and our business, financial condition and results of operations may be adversely affected.
The results of pre-clinical studies and completed clinical trials are not necessarily predictive of future results, and our current product candidates may not have favorable results in later studies or trials which, in turn, could have a material adverse effect on our business.
As described above, we must conduct extensive testing of our product candidates and new indications of our marketed products before we can obtain regulatory approval to market and sell them. Success in pre-clinical studies or completed clinical trials does not ensure that later studies or trials, including continuing pre-clinical studies and large-scale clinical trials, will be successful nor does it necessarily predict future results. Favorable results in early studies or trials may not be repeated in later studies or trials, and product candidates in later stage trials may fail to show acceptable safety and efficacy despite having progressed through earlier trials. We may be required to demonstrate through large, long-term outcome trials that our product candidates are safe and effective for use in a broad population prior to obtaining regulatory approval. The failure of clinical trials to demonstrate the safety and effectiveness of our clinical candidates for the desired indication(s) would preclude the successful development of those candidates for such indication(s), in which event our business, prospects, results of operations and financial condition may be adversely affected.
Our failure to obtain necessary regulatory clearances or approvals on a timely basis would significantly impair our ability to distribute and market our future products on the clinical IVD market.
We are subject to regulation by the FDA in the United States, the Conformité Européenne in Europe and other regulatory bodies in other countries where we intend to sell our future products. Before we are able to place our intended products in the clinical IVD markets in the United States and Europe, we will be required to obtain clearance or approval of our future products from the FDA and receive a CE Mark, respectively.
12
The European Union has recently adopted regulations that may impose additional requirements to obtain a CE Mark, which could result in delays and further expense, in terms of staff costs to us as compared to the current CE Mark process. The new regulations will require each product submission to be thoroughly audited by Notified Bodies, instead of the current self-certification process. The EU MDR will be fully applicable in 2020 and the EU IVDR will be fully applicable in 2022.
Additionally, even if we receive the required government clearance or approval of our intended products, we are still subject to continuing regulation and oversight. Under the FDA, diagnostics are considered medical devices and are subject to ongoing controls and regulations, including inspections, compliance with established manufacturing practices, device-tracking, record-keeping, advertising, labeling, packaging, and compliance with other standards. The process of complying with such regulations with respect to current and new products can be costly and time-consuming. Failure to comply with these regulations could have a material adverse effect on our business, financial condition, and results of operations. Furthermore, any FDA regulations governing our future products are subject to change at any time, which may cause delays and have material adverse effects on our operations. In Europe, IVD companies are currently able to self-certify that they meet the appropriate regulatory requirements (which is subject to change with the EU MDR and the EU IVDR noted above) but are subject to inspection for enforcement. European national agencies, such as customs authorities and/or the Departments of Health, Industry and Labor, conduct market surveillance to ensure the applicable requirements have been met for products marketed within the European Union.
Reductions or changes in reimbursement policies could limit our ability to sell our products.
Market acceptance and sales of our products will depend, in part, on reimbursement policies and may be affected by healthcare reform measures. Government authorities and third-party payors, such as private health insurers and health maintenance organizations, decide which products they will pay for and establish reimbursement levels for those products. To manage healthcare costs, many governments and third-party payors in the United States increasingly scrutinize the pricing of new products and require greater levels of evidence of favorable clinical outcomes and cost-effectiveness before extending coverage. We cannot be sure that reimbursement will be available for our products and, if reimbursement is available, the level of such reimbursement. Reimbursement may impact the demand for, or the price of, our products. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize our future products.
If the marketplace does not accept the products in our development pipeline or any other diagnostic products we might develop, we may be unable to generate sufficient revenue to sustain and grow our business.
Our intended products may never gain significant acceptance in the research or clinical marketplace and therefore may never generate substantial revenue or profits. Physicians, hospitals, clinical laboratories, researchers or others in the healthcare industry may not use our future products unless they determine that they are an effective and cost-efficient means of detecting and diagnosing cancer. If our research and studies do not satisfy providers, payors and others as to the reliability and effectiveness, we may experience reluctance or refusal on the part of the physician to use our future products. In addition, we will need to expend a significant amount of resources on marketing and educational efforts to create awareness of our future products and to encourage their acceptance and adoption. If the market for our future products does not develop sufficiently or the products are not accepted, our revenue potential will be harmed.
The cancer diagnostics market is highly competitive and subject to rapid technological change; accordingly, we will face fierce competition and our intended products may become obsolete.
The cancer diagnostics market is extremely competitive and characterized by evolving industry standards and new product enhancements. Cancer diagnostic tests are technologically innovative and require significant planning, design, development, and testing at the technological, product, and manufacturing process levels. These activities require significant capital commitments and investment. There can be no assurance that our intended products or proprietary technologies will remain competitive following the introduction of new products and technologies by competing companies within the industry. Furthermore, there can be no assurance that our competitors will not develop products that render our future products obsolete or that are more effective, accurate or can be produced at lower costs. There can be no assurance that we will be successful in the face of increasing competition from new technologies or products introduced by existing companies in the industry or by new companies entering the market.
We expect to face intense competition from companies with greater resources and experience than us, which may increase the difficulty for us to achieve significant market penetration.
The market for cancer diagnostics is intensely competitive, subject to rapid change, and significantly affected by new product introductions and other market activities of industry participants. Our competitors include large multinational corporations and their operating units, including Abbott Laboratories Inc., Cepheid Inc., Philips, GE Healthcare, Siemens, Gen-Probe Incorporated, MDxHealth SA, EpiGenomics AG, Roche Diagnostics, Exact Sciences Corporation and several others. Most of these companies have substantially greater financial, marketing and other resources than we do. Most of these companies are either publicly traded or a division of a publicly traded company, and enjoy several competitive advantages, including:
13
significantly greater name recognition;
established relationships with healthcare professionals, companies and consumers;
additional lines of products, and the ability to offer rebates or bundle products to offer higher discounts or incentives to gain a competitive advantage;
established supply and distribution networks; and
greater resources for product development, sales and marketing, and intellectual property protection.
Many of these other companies have developed and will continue to develop new products that will compete directly with our future products. In addition, many of our competitors spend significantly greater funds for the research, development, promotion, and sale of new and existing products. These resources may allow them to respond more quickly to new or emerging technologies and changes in consumer requirements. We also face competition in our search for third parties to assist us with sales and marketing and our product candidates, which may negatively impact our ability to enter into favorable sales and marketing arrangements. For all the foregoing reasons, we may not be able to compete successfully against our competitors.
Declining global economic or business conditions may have a negative impact on our business.
Continuing concerns over United States healthcare reform legislation and energy costs, geopolitical issues, the availability and cost of credit and government stimulus programs in the United States and other countries have contributed to increased volatility and diminished expectations for the global economy. These factors, combined with low business and consumer confidence and high unemployment precipitated a global economic slowdown and recession. If the economic climate does not improve or continues to deteriorate, our business, including our access to the RUO or clinical IVD markets for diagnostic tests, could be adversely affected, resulting in a negative impact on our business, financial condition and results of operations.
On June 23, 2016, the United Kingdom held a referendum in which voters approved an exit from the European Union, commonly referred to as “Brexit”. As a result of the referendum, the British government has begun to negotiate the terms of the United Kingdom’s future relationship with the European Union. Although it is unknown what those terms will be, it is possible that there will be greater restrictions on imports and exports between the European Union countries and the United Kingdom and increased regulatory complexities. These changes may adversely affect our ability to market our future products in the United Kingdom which could have an adverse effect on our business, financial condition, and results of operations.
We will rely on third parties to manufacture and supply our intended products. Any problems experienced by these third parties could result in a delay or interruption in the supply of our intended products to our customers, which could have a material negative effect on our business.
We will rely on third parties to manufacture and supply our intended products. The manufacture of our intended diagnostic products will require specialized equipment and utilize complicated production processes that would be difficult, time-consuming and costly to duplicate. If the operations of third party manufacturers are interrupted or if they are unable to meet our delivery requirements due to capacity limitations or other constraints, we may be limited in our ability to fulfill our future sales orders. Any prolonged disruption in the operations of third party manufacturers could have a significant negative impact on our ability to sell our future products, could harm our reputation and could cause us to seek other third party manufacturing contracts, thereby increasing our anticipated development and commercialization costs. In addition, if we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards required by the FDA and with all applicable regulations and guidelines. The delays associated with the verification of a new manufacturer could negatively affect our ability to develop products or receive approval of any products in a timely manner.
The manufacturing operations of our future third party manufacturers will likely be dependent upon third party suppliers, making us vulnerable to supply shortages and price fluctuations, which could harm our business.
The operations of our future third party manufacturers will likely be dependent upon third party suppliers. A supply interruption or an increase in demand beyond a supplier’s capabilities could harm the ability of our future manufacturers to manufacture our intended products until new sources of supply are identified and qualified.
14
Reliance on these suppliers could subject us to a number of risks that could harm our business, including:
interruption of supply resulting from modifications to or discontinuation of a supplier’s operations;
delays in product shipments resulting from uncorrected defects, reliability issues, or a supplier’s variation in a component;
a lack of long-term supply arrangements for key components with our suppliers;
inability to obtain adequate supply in a timely manner, or to obtain adequate supply on commercially reasonable terms;
difficulty and cost associated with locating and qualifying alternative suppliers for components in a timely manner;
production delays related to the evaluation and testing of products from alternative suppliers, and corresponding regulatory qualifications;
delay in delivery due to suppliers prioritizing other customer orders over ours;
damage to our brand reputation caused by defective components produced by the suppliers; and
fluctuation in delivery by the suppliers due to changes in demand from us or their other customers.
Any interruption in the supply of components of our future products or materials, or our inability to obtain substitute components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our future customers, which would have an adverse effect on our business.
We will depend on third party distributors in the future to market and sell our future products which will subject us to a number of risks.
We will depend on third party distributors to sell, market, and service our future products in our intended markets. We are subject to a number of risks associated with reliance upon third party distributors including:
lack of day-to-day control over the activities of third party distributors;
third party distributors may not commit the necessary resources to market and sell our future products to our level of expectations;
third party distributors may terminate their arrangements with us on limited or no notice or may change the terms of these arrangements in a manner unfavorable to us; and
disagreements with our future distributors could result in costly and time-consuming litigation or arbitration which we could be required to conduct in jurisdictions with which we are not familiar.
If we fail to establish and maintain satisfactory relationships with our future third party distributors, our revenues and market share may not grow as anticipated, and we could be subject to unexpected costs which could harm our results of operations and financial condition.
If the patents that we rely on to protect our intellectual property prove to be inadequate, our ability to successfully commercialize our future products will be harmed and we may never be able to operate our business profitably.
Our success depends, in large part, on our ability to protect proprietary methods, discoveries and technologies that we develop under the patents and intellectual property laws of the United States, the European Union and other countries, so that we can seek to prevent others from unlawfully using our inventions and proprietary information. We have 17 patent families related to our diagnostic tests, with five patents granted in the United States and four patents granted in the European Union. Additionally, we have 12 patent applications in the name of our subsidiaries pending in the United States and the 13 patent applications in the European Union.
If we are not able to protect our proprietary technology and information, our competitors may use our inventions to develop competing products. We cannot assure you that any of the pending patent applications will result in patents being issued. In addition, due to technological changes that may affect our future products or judicial interpretation of the scope of our patents, our intended products might not, now or in the future, be adequately covered by our patents.
If third parties assert that we have infringed their patents and proprietary rights or challenge the validity of our patents and proprietary rights, we may become involved in intellectual property disputes and litigation that would be costly, time consuming, and delay or prevent the development or commercialization of our future products.
Our ability to commercialize our intended products depends on our ability to develop, manufacture, market and sell our future products without infringing the proprietary rights of third parties. Third parties may allege that our future products or our methods or discoveries infringe their intellectual property rights. Numerous United States and foreign patents and pending patent applications, which are owned by third parties, exist in fields that relate to our intended products and our underlying methodologies, discoveries and technologies. A third party may sue us for infringing its patent rights.
15
Our ability to successfully commercialize our intended products depends on our ability to protect our proprietary technology and information. Likewise, we may need to resort to litigation to enforce a patent issued or licensed to us or to determine the scope and validity of third party proprietary rights. In addition, a third party may claim that we have improperly obtained or used its confidential or proprietary information. The cost to us of any litigation or other proceeding relating to intellectual property rights, even if resolved in our favor, could be substantial, and the litigation could divert our management’s attention from other aspects of our business. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of any litigation could limit our ability to continue our operations. Additionally, we cannot be certain of the level of protection, if any, that will be provided by our patents if they are challenged in court, where our competitors may raise defenses such as invalidity, unenforceability or possession of a valid license.
If we are found to infringe upon intellectual property rights of third parties, we might be forced to pay damages, potentially including treble damages. In addition to any damages we might have to pay, a court could require us to stop the infringing activity or obtain a license. Any license required under any patent may not be made available on commercially acceptable terms, if at all. In addition, such licenses are likely to be non-exclusive and, therefore, our competitors may have access to the same technology licensed to us. If we fail to obtain a required license and are unable to design around a patent, we may be unable to effectively market some or all of our future products, which could limit our ability to generate revenue or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations.
If we are unable to protect our trade secrets, we may be unable to protect our interests in proprietary technology, processes and know-how that is not patentable or for which we have elected not to seek patent protection.
In addition to patented technology, we rely upon trade secret protection to protect our interests in proprietary know-how and for processes for which patents are difficult or impossible to obtain or enforce. We may not be able to protect our trade secrets adequately. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors and outside scientific advisors may unintentionally or willfully disclose our information to competitors. Enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets. We rely, in part, on non-disclosure and confidentiality agreements with our employees, consultants and other parties to protect our trade secrets and other proprietary technology. These agreements may be breached and we may not have adequate remedies for any breach. Moreover, others may independently develop equivalent proprietary information, and third parties may otherwise gain access to our trade secrets and proprietary knowledge. Any disclosure of confidential information into the public domain or to third parties could allow our competitors to learn our trade secrets and use the information in competition against us, which could adversely affect our competitive advantage.
Defects in our products may subject us to substantial damages which could materially harm our business or financial condition.
The products we develop could lead to product liability claims based on allegations that one or more of our products contained a design or manufacturing defect which resulted in the failure to detect the disease for which it was designed. A product liability claim could result in substantial damages and be costly and time consuming to defend, either of which could materially harm our business or financial condition. We cannot assure you that our product liability insurance would protect our assets from the financial impact of defending a product liability claim. Any product liability claim brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing insurance coverage in the future.
16
Risks Associated with our Common Stock
The market prices and trading volume of our stock may be volatile.
The market price of our common stock is likely to be highly volatile and the trading volume may fluctuate and cause significant price variation to occur. We cannot assure you that the market prices of our common stock will not fluctuate or decline significantly in the future. Some of the factors that could negatively affect the prices of our shares or result in fluctuations in those prices or in trading volume of our common stock could include the following, many of which will be beyond our control:
competition;
comments by securities analysts regarding our business or prospects;
additions or departures of key personnel;
our ability to execute our business plan;
issuance of common stock or other securities;
operating results that fall below expectations;
loss of any strategic relationship;
industry developments;
economic and other external factors; and
period-to-period fluctuations in our financial results.
In addition, the securities markets have from time to time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price and trading volume of our common stock.
Share ownership by our executive officers and directors make it more difficult for third parties to acquire us or effectuate a change of control that might be viewed favorably by other stockholders.
As of February 27, 2018, our executive officers and directors beneficially owned, in the aggregate, approximately 29.9% of our outstanding shares. As a result, if the executive officers and directors were to oppose a third party’s acquisition proposal for, or a change in control of, the Company, such officers and directors may have sufficient voting power to be able to block or at least delay such an acquisition or change in control from taking place, even if other stockholders would support such a sale or change of control.
Our corporate governance documents, and certain corporate laws applicable to us, could make a takeover attempt, which may be beneficial to our stockholders, more difficult.
Our Board of Directors, or Board, has the power, under our charter documents to:
issue additional shares of common stock without having to obtain stockholder approval for such action;
enable our Board to fill vacant directorships except for vacancies created by the removal of a director;
enable our Board to amend our bylaws without stockholder approval subject to certain exceptions; and
require compliance with an advance notice procedure with regard to business to be brought by a stockholder before an annual or special meeting of stockholders and with regard to the nomination by stockholders of candidates for election as directors.
These provisions may discourage potential acquisition proposals and could delay or prevent a change of control, including under circumstances in which our stockholders might otherwise receive a premium over the market price of our common stock.
We do not expect to pay dividends in the foreseeable future.
We have never declared or paid cash dividends on our common stock. We do not intend to declare dividends for the foreseeable future, as we anticipate that we will reinvest any future earnings in the development and growth of our business. Therefore, investors will not receive any funds unless they sell their common stock, and stockholders may be unable to sell their shares on favorable terms or at all. We cannot assure you of a positive return on investment or that you will not lose the entire amount of your investment in our common stock.
17
We may in the future issue additional shares of our common stock which would reduce investors’ ownership interests in the Company and which may cause our stock price to decline.
Our Second Amended and Restated Certificate of Incorporation and amendments thereto authorize the issuance of 100,000,000 shares of common stock, par value $0.001 per share. The future issuance of all or part of our remaining authorized common stock may result in substantial dilution in the percentage of our common stock held by our then existing stockholders. We may value any common stock issued in the future on an arbitrary basis. The issuance of common stock for future services or acquisitions or other corporate actions may have the effect of diluting the percentage ownership of our stockholders and, depending upon the prices at which such shares are sold or issued, on their investment in our common stock and, therefore, could have an adverse effect on any trading market for our common stock.
Future sales of our common stock could depress the market price of our common stock.
Sales of a substantial number of shares of our common stock in the public market or the perception that large sales of our shares could occur, could cause the market price of our common stock to decline or limit our future ability to raise capital through an offering of equity securities.
If equity research analysts do not publish research or reports about our business, or if they do publish such reports but issue unfavorable commentary or downgrade our common stock, the price and trading volume of our common stock could decline.
The trading market for our common stock could be affected by whether and to what extent equity research analysts publish research or reports about us and our business. If one or more equity analysts cover us and publish research reports about our common stock, the price of our stock could decline rapidly if one or more securities analysts downgrade our stock or if those analysts issue or offer unfavorable commentary or cease publishing reports about us. If any of these analysts ceases coverage of us, we could lose visibility in the market, which in turn could cause our common stock price or trading volume to decline and our common stock to be less liquid.
We are a smaller reporting company and we cannot be certain if the reduced disclosure requirements applicable to smaller reporting companies will make our common stock less attractive to investors.
We are currently a “smaller reporting company,” meaning that we are not an investment company, an asset-backed issuer, or a majority-owned subsidiary of a parent company that is not a smaller reporting company and have a public float of less than $75 million measured as of the last business day of our most recently completed second fiscal quarter and annual revenues of less than $50 million during the most recently completed fiscal year. “Smaller reporting companies” are able to provide simplified executive compensation disclosures in their filings; are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting; and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. Decreased disclosures in our SEC filings due to our status as a “smaller reporting company” may make it harder for investors to analyze our results of operations and financial prospects.
18
ITEM 1B.UNRESOLVED STAFF COMMENTS
None.
ITEM 2.PROPERTIES
As of December 31, 2017, we occupied approximately 17,300 square feet at our significant laboratory facility in Namur, Belgium, which facility we acquired in October 2016. The purchase price for the property was €1.2 million Euros, exclusive of any closing costs (See Note 10(c) to the Consolidated Financial Statements).
Listed below are our current facilities:
|
Location |
Primary Function |
Approx. Square Feet |
Leased or Owned |
|
Namur, Belgium |
Research and development |
17,300 |
Owned |
|
London, UK (1) |
Sales and marketing |
690 |
Leased, expiring 2019 |
|
Shaw Centre, Singapore (2) |
Executive suite |
150 |
Leased, expiring 2018 |
(1)Volition Diagnostics UK signed a fixed two-year lease for this property located at 93-95 Gloucester Place, London, W1U 6JQ, United Kingdom, commencing January 30, 2017, at an annual rent of £110,400 GBP.
(2)Singapore Volition signed a one-year lease for this property, commencing August 1, 2017, located at 1 Scotts Road, #24-05 Shaw Centre, Singapore 228208, at an annual rent of SGD 29,519.
ITEM 3.LEGAL PROCEEDINGS
In the ordinary course of business, we may be subject to claims, counter claims, suits and other litigation of the type that generally arise from the conduct of our business. We are not aware of any threatened or pending litigation that we expect will have a material adverse effect on our business operations, financial condition or results of operations.
ITEM 4.MINE SAFETY DISCLOSURES
Not Applicable.
19
ITEM 5.MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES
Market Information
Our common stock is currently traded on the NYSE American under the symbol “VNRX”. The following table provides for the periods indicated the high and low sales prices per share as reported on the NYSE American.
|
2017 |
High |
Low |
|
First quarter |
$5.45 |
$4.00 |
|
Second quarter |
$4.20 |
$2.92 |
|
Third Quarter |
$3.59 |
$2.45 |
|
Fourth Quarter |
$3.75 |
$2.08 |
|
|
|
|
|
2016 |
High |
Low |
|
First quarter |
$4.43 |
$3.20 |
|
Second quarter |
$4.19 |
$3.05 |
|
Third quarter |
$5.86 |
$3.05 |
|
Fourth quarter |
$5.39 |
$3.75 |
As at February 27, 2018, there were 26,530,793 shares of our common stock outstanding held by 168 holders of record, based on information provided by our transfer agent.
Dividends
We have not declared or paid any cash dividends on our common stock since inception and presently anticipate that all earnings, if any, will be retained for development of our business and that no dividends on our common stock will be declared in the foreseeable future. Any future dividends will be subject to the discretion of our board of directors and will depend upon, among other things, future earnings, operating and financial conditions, capital requirements, general business conditions and other pertinent facts. Therefore, there can be no assurance that any dividends on our common stock will be paid in the future.
Securities Authorized for Issuance Under Equity Compensation Plans
See “Securities Authorized for Issuance Under Equity Compensation Plans” included under Part III, Item 12 of this report, which is incorporated by reference into this Item 5.
Recent Sales of Unregistered Securities
None.
Repurchase of Equity Securities
None.
ITEM 6.SELECTED FINANCIAL DATA
We are currently a smaller reporting company and are not required to disclose this information.
20
ITEM 7.MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
Overview
We have identified the specific processes and resources required to achieve the near and medium term objectives of our business plan, including personnel, facilities, equipment, research and testing materials including antibodies and clinical samples, and the protection of intellectual property. To date, operations have proceeded satisfactorily in relation to our business plan. However it is possible that some resources will not readily become available in a suitable form or on a timely basis or at an acceptable cost. It is also possible that the results of some processes may not be as expected and that modifications of procedures and materials may be required. Such events could result in delays to the achievement of the near and medium term objectives of our business plan, in particular the progression of clinical validation studies and regulatory approval processes for the purpose of bringing products to the IVD market.
Our future as an operating business will depend on our ability to obtain sufficient capital contributions, financing and/or generate revenues as may be required to sustain our operations. Management plans to address the above as needed by: (a) securing additional grant funds; (b) obtaining additional equity or debt financing; (c) grants of licensing rights to third parties in exchange for specified up-front and/or back end payments; and (d) developing and commercializing our products on an accelerated timeline. Management continues to exercise tight cost controls to conserve cash.
Our ability to continue as a going concern is dependent upon our accomplishment of the plans described in the preceding paragraph and eventually to attain profitable operations. The accompanying financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern. If we are unable to obtain adequate capital, we could be forced to cease operations.
Liquidity and Capital Resources
We have financed our operations since inception primarily through private placements and public offerings of our common stock. As of December 31, 2017, we had cash and cash equivalents of approximately $10.1 million.
For the year ended December 31, 2017, we used approximately $12.2 million in net cash from operating activities, compared to $8.9 million for the year ended December 31, 2016. The increase in cash used year over year was primarily due to increased expenditures on research and development activities, as well as increased general and administrative activities, including increases in stock-based compensation and personnel expenses.
Net cash used in investing activities was $1.4 million and $0.4 million for the years ended December 31, 2017 and 2016, respectively. The increase in cash used in investing activities for the year ended December 31, 2017 when compared to same period in 2016 was primarily a result of the purchase of equipment and facility improvements for the new research and development facility in Belgium and investment in our information technology infrastructure.
Net cash provided by financing activities was approximately $2.0 million for the year ended December 31, 2017, compared to $25.2 million for the year ended December 31, 2016. The decrease in cash provided by financing activities was primarily the result of a significant decrease in capital raising activities during the year ended December 31, 2017 offset by an increase in debt financing as compared to the prior year period.
The following table summarizes our approximate contractual payments due by period as of December 31, 2017:
|
Approximate Payments Due by Period | ||||||||
|
|
|
Total |
|
Less Than One Year |
|
2 - 5 Years |
|
More than 5 years |
|
Description |
|
$ |
|
$ |
|
$ |
|
$ |
|
Capital Lease Commitments |
|
1,171,310 |
|
163,100 |
|
404,118 |
|
604,092 |
|
Loans Repayable(1) |
|
3,236,515 |
|
536,231 |
|
1,928,637 |
|
771,647 |
|
Operating Leases |
|
343,405 |
|
214,152 |
|
129,253 |
|
- |
|
|
|
|
|
|
|
|
|
|
|
Collaborative Agreements |
|
|
|
|
|
|
|
|
|
Denmark |
|
1,034,630 |
|
1,034,630 |
|
- |
|
- |
|
Munich |
|
431,294 |
|
143,764 |
|
287,530 |
|
- |
|
University of Michigan |
|
2,500,000 |
|
1,000,000 |
|
1,500,000 |
|
- |
|
Nat University of Singapore |
|
9,600 |
|
9,600 |
|
- |
|
- |
|
Total |
|
8,726,754 |
|
3,101,477 |
|
4,249,538 |
|
1,375,739 |
(1)Loans Repayable includes the total value of the SOFINEX loan of €1.0 million Euros. See Note 10(k) to the Consolidated Financial Statements for the details.
21
We intend to use our cash reserves to predominantly fund further research and development activities. We do not currently have any substantial source of revenues and expect to rely upon additional financing to fully-fund our current strategic plan, which includes successfully commercializing a suite of diagnostic tests as well as additional products. We can provide no assurance that we will be successful in raising further funds.
In the event that additional financing is delayed, we will prioritize the maintenance of our research and development personnel and facilities, primarily in Belgium, and the maintenance of our patent rights. The completion of clinical validation studies and regulatory approval processes for the purpose of bringing products to the IVD market would be delayed. In the event of an ongoing lack of financing, it may be necessary to discontinue operations, which will adversely affect the value of our common stock.
Results of Operations
Comparison of the Years Ended December 31, 2017 and December 31, 2016.
The following table sets forth our results of operations for the year ended on December 31, 2017 and the comparative period for the year ended December 31, 2016.
|
|
|
Year |
|
Year |
|
|
|
|
|
|
|
Ended |
|
Ended |
|
|
|
Percentage |
|
|
|
December 31, |
|
December 31, |
|
Increase/ |
|
Increase/ |
|
|
|
2017 |
|
2016 |
|
(Decrease) |
|
(Decrease) |
|
|
|
$ |
|
$ |
|
$ |
|
% |
|
Revenues |
|
- |
|
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
(8,906,006) |
|
(7,904,988) |
|
1,001,018 |
|
13% |
|
|
(5,376,500) |
|
(4,177,513) |
|
1,198,987 |
|
29% |
|
|
Sales and marketing |
|
(763,407) |
|
(347,712) |
|
415,695 |
|
120% |
|
|
|
|
|
|
|
|
|
|
|
Total Operating Expenses |
|
(15,045,913) |
|
(12,430,213) |
|
2,615,700 |
|
21% |
|
|
|
|
|
|
|
|
|
|
|
Other income |
|
349,839 |
|
545,313 |
|
(195,474) |
|
(36%) |
|
Interest expense |
|
(73,133) |
|
(20,378) |
|
52,755 |
|
259% |
|
Total Other Income |
|
276,706 |
|
524,935 |
|
(248,229) |
|
(47%) |
|
|
|
|
|
|
|
|
|
|
|
Income Taxes |
|
- |
|
- |
|
- |
|
- |
|
|
|
|
|
|
|
|
|
|
|
Net Loss |
|
(14,769,207) |
|
(11,905,278) |
|
2,863,929 |
|
24% |
|
|
|
|
|
|
|
|
|
|
|
Net Loss Per Share – Basic and Diluted |
|
(0.56) |
|
(0.52) |
|
0.04 |
|
8% |
|
|
|
|
|
|
|
|
|
|
|
Weighted Average Shares Outstanding - Basic and Diluted |
|
26,389,580 |
|
23,049,089 |
|
3,340,491 |
|
14% |
Revenues
Our operations are still predominantly in the development stage and we had no revenues during the years ended December 31, 2017 or 2016.
Operating Expenses
Total operating expenses increased to $15.0 million for the year ended December 31, 2017 from $12.4 million for the year ended December 31, 2016.
22
Research and Development Expenses
Research and development expenses increased to $8.9 million for the year ended December 31, 2017 from $7.9 million for the year ended December 31, 2016. This increase in overall research and development expenditures was primarily related to additional costs of $0.5 million due our participation in the 13,500 patient trial with the National Cancer Institute’s Early Detection Research Network in collaboration with the University of Michigan.
|
|
2017 |
|
2016 |
|
Change |
|
|
$ |
|
$ |
|
$ |
|
Personnel expenses |
3,707,202 |
|
3,516,007 |
|
191,195 |
|
Research collaboration |
2,340,650 |
|
2,109,300 |
|
231,350 |
|
Direct research and development expenses |
558,605 |
|
829,029 |
|
(270,424) |
|
Legal and professional fees |
1,788,206 |
|
1,137,575 |
|
650,631 |
|
Depreciation and amortization |
511,343 |
|
313,077 |
|
198,266 |
|
Total Research and Development expenses |
8,906,006 |
|
7,904,988 |
|
1,001,018 |
General and Administrative Expenses
General and administrative expenses increased to $5.4 million for year ended December 31, 2017 from $4.2 million for the year ended December 31, 2016. The increase was primarily related to increase in stock-based compensation and increased personnel costs.
|
2017 |
|
2016 |
|
Change |
|
|
|
$ |
|
$ |
|
$ |
|
Personnel expenses |
1,076,791 |
|
705,321 |
|
371,470 |
|
Stock-based compensation |
2,376,041 |
|
1,809,828 |
|
566,213 |
|
Legal and professional fees |
1,779,659 |
|
1,608,568 |
|
171,091 |
|
Information technology support costs |
129,576 |
|
53,796 |
|
75,780 |
|
Depreciation and amortization |
14,433 |
|
- |
|
14,433 |
|
Total General and Administrative expenses |
5,376,500 |
|
4,177,513 |
|
1,198,987 |
Sales and marketing expenses increased to $0.8 million for year ended December 31, 2017 from $0.3 million for the year ended December 31, 2016. The increase was primarily related to increased personnel and increased stock-based compensation.
|
|
2017 |
|
2016 |
|
Change |
|
|
$ |
|
$ |
|
$ |
|
Personnel expenses |
426,541 |
|
64,769 |
|
361,772 |
|
Stock-based compensation |
113,557 |
|
33,093 |
|
80,464 |
|
Other sales and marketing expenses |
223,309 |
|
249,850 |
|
(26,541) |
|
Total Sales and Marketing expenses |
763,407 |
|
347,712 |
|
415,695 |
Other income decreased to $0.3 million for the year ended December 31, 2017 from $0.5 million for the year ended December 31, 2016. The decrease was due to less grant funds received from public bodies in respect of approved expenditures, where there is no obligation to repay in 2017 compared to 2016.
Net loss increased to $14.8 million for the year ended December 31, 2017 from $11.9 million for the year ended December 31, 2016. The change resulted from the factors described above.
We have not attained profitable operations and are dependent upon obtaining external financing to continue to pursue our operational and strategic plans. For these reasons, management has determined that there is substantial doubt that the business will be able to continue as a going concern without further financing.
23
Off-Balance Sheet Arrangements
We have no significant off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, collaborative agreements, liquidity, capital expenditures or capital resources that are material to stockholders.
Our financial statements and accompanying notes have been prepared in accordance with United States generally accepted accounting principles, or U.S. GAAP, applied on a consistent basis. The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods.
We regularly evaluate the accounting policies and estimates that we use to prepare our financial statements. A complete summary of these policies is included in the notes to our financial statements. In general, management’s estimates are based on historical experience, on information from third party professionals, and on various other assumptions that are believed to be reasonable under the facts and circumstances. Actual results could differ from those estimates made by management.
We consider the following accounting policies to be critical:
Stock-Based Compensation
The Company records stock-based compensation in accordance with ASC 718, Compensation – Stock Compensation and ASC 505-50, Equity-Based Payments to Non-Employees. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. Equity instruments issued to employees and the cost of the services received as consideration are measured and recognized based on the fair value of the equity instruments issued and are recognized over the employees required service period, which is generally the vesting period.
Impairment of Long-Lived Assets
In accordance with ASC 360, Property Plant and Equipment, the Company tests long-lived assets or asset groups for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. Circumstances which could trigger a review include, but are not limited to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; and current expectation that the asset will more likely than not be sold or disposed of significantly before the end of its estimated useful life. Recoverability is assessed based on the carrying amount of the asset and its fair value which is generally determined based on the sum of the undiscounted cash flows expected to result from the use and the eventual disposal of the asset, as well as specific appraisal in certain instances. An impairment loss is recognized when the carrying amount is not recoverable and exceeds fair value. Impairment losses of $nil and $nil were recognized during the years ended December 31, 2017 and December 31, 2016, respectively.
Foreign Currency Translation
The Company has functional currencies in the Euro, the United States Dollar and British Pounds Sterling and its reporting currency is the United States Dollar. Management has adopted ASC 830-20, “Foreign Currency Matters – Foreign Currency Transactions”. All assets and liabilities denominated in foreign currencies are translated using the exchange rate prevailing at the balance sheet date. For revenues and expenses, the weighted average exchange rate for the period is used. Gains and losses arising on translation of foreign currency denominated transactions are included in Other Comprehensive Income (Loss).
Recently Issued Accounting Pronouncements
We have implemented all new accounting pronouncements that are in effect and applicable to us. We do not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on our financial position or results of operations.
ITEM 7A.QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK
We are currently a smaller reporting company and are not required to disclose this information.
24
ITEM 8. FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA
VOLITIONRX LIMITED
Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
|
|
Index |
|
|
|
|
Report of Independent Registered Public Accounting Firm |
F-2 |
|
|
|
|
Consolidated Balance Sheets |
F-3 |
|
|
|
|
Consolidated Statements of Operations and Comprehensive Loss |
F-4 |
|
|
|
|
Consolidated Statements of Cash Flows |
F-5 |
|
|
|
|
Consolidated Statement of Stockholders’ Equity |
F-7 |
|
|
|
|
Notes to the Consolidated Financial Statements |
F-8 |
F-1

REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of VolitionRX Limited:
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of VolitionRX Limited (“the Company”) as of December 31, 2017 and 2016, and the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the years in the two-year period ended December 31, 2017 and the related notes (collectively referred to as the “consolidated financial statements”). In our opinion, the consolidated financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2017, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph Regarding Going Concern
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the consolidated financial statements, the Company has suffered net losses since inception and has accumulated a significant deficit. These factors raise substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. Federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our Audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
/s/ Sadler, Gibb & Associates, LLC
We have served as the Company’s auditor since 2011.
Salt Lake City, UT
March 1, 2018
office801.783.2950
fax801.783.2960
www.sadlergibb.com / Main: 2455 East Parley’s Way, Suite 320, Salt Lake City, UT 84109 / Provo: 3507 N University Ave #100, Provo UT 84604
F-2
VOLITIONRX LIMITED
Consolidated Balance Sheets
(Expressed in United States Dollars, except share numbers)
|
|
|
December 31, 2017 $ |
|
December 31, 2016 $ |
|
|
|
|
|
|
|
ASSETS |
|
|
|
|
|
|
|
|
|
|
|
Cash and cash equivalents |
|
10,116,263 |
|
21,678,734 |
|
Prepaid expenses |
|
248,661 |
|
165,927 |
|
Other current assets |
|
202,295 |
|
166,887 |
|
|
|
|
|
|
|
Total Current Assets |
|
10,567,219 |
|
22,011,548 |
|
|
|
|
|
|
|
Property and equipment, net |
|
3,480,782 |
|
2,119,027 |
|
Intangible assets, net |
|
576,397 |
|
602,193 |
|
|
|
|
|
|
|
Total Assets |
|
14,624,398 |
|
24,732,768 |
|
|
|
|
|
|
|
LIABILITIES |
|
|
|
|
|
|
|
|
|
|
|
Accounts payable |
|
351,735 |
|
281,179 |
|
Accrued liabilities |
|
1,278,428 |
|
1,439,275 |
|
Management and directors’ fees payable |
|
35,397 |
|
81,057 |
|
Current portion of long-term debt |
|
443,908 |
|
30,655 |
|
Current portion of capital lease liabilities |
|
139,084 |
|
119,016 |
|
Deferred grant income |
|
- |
|
45,510 |
|
Current portion of grant repayable |
|
41,930 |
|
36,804 |
|
|
|
|
|
|
|
Total Current Liabilities |
|
2,290,482 |
|
2,033,496 |
|
|
|
|
|
|
|
Long-term debt |
|
1,312,785 |
|
432,027 |
|
Capital lease liabilities |
|
874,684 |
|
889,810 |
|
Grant repayable |
|
188,579 |
|
202,325 |
|
|
|
|
|
|
|
Total Liabilities |
|
4,666,530 |
|
3,557,658 |
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY |
|
|
|
|
|
Common Stock Authorized: 100,000,000 shares, at $0.001 par value Issued and outstanding: 26,519,394 shares and 26,126,049 shares, respectively |
|
26,519 |
|
26,126 |
|
Additional paid-in capital |
|
65,774,870 |
|
62,287,252 |
|
Accumulated other comprehensive loss |
|
(129,343) |
|
(193,297) |
|
Accumulated Deficit |
|
(55,714,178) |
|
(40,944,971) |
|
|
|
|
|
|
|
Total Stockholders’ Equity |
|
9,957,868 |
|
21,175,110 |
|
|
|
|
|
|
|
Total Liabilities and Stockholders’ Equity |
|
14,624,398 |
|
24,732,768 |
|
|
|
|
|
|
(The accompanying notes are an integral part of these consolidated financial statements)
F-3
VOLITIONRX LIMITED
Consolidated Statements of Operations and Comprehensive Loss
(Expressed in United States Dollars, except share numbers)
|
|
For the year ended December 31, 2017 $ |
|
For the year ended December 31, 2016 $ |
|
|
|
|
|
|
Revenue |
- |
|
- |
|
|
|
|
|
|
Operating Expenses |
|
|
|
|
|
|
|
|
|
Research and development |
8,906,006 |
|
7,904,988 |
|
General and administrative |
5,376,500 |
|
4,177,513 |
|
Sales and marketing |
763,407 |
|
347,712 |
|
|
|
|
|
|
Total Operating Expenses |
15,045,913 |
|
12,430,213 |
|
|
|
|
|
|
Operating Loss |
(15,045,913) |
|
(12,430,213) |
|
Other Income (Expenses) |
|
|
|
|
Other income |
349,839 |
|
545,313 |
|
Interest expense |
(73,133) |
|
(20,378) |
|
|
|
|
|
|
Total Other Income |
276,706 |
|
524,935 |
|
|
|
|
|
|
Provision for Income Taxes |
- |
|
- |
|
|
|
|
|
|
Net Loss |
(14,769,207) |
|
(11,905,278) |
|
|
|
|
|
|
Other Comprehensive Income (Loss) |
|
|
|
|
Foreign currency translation adjustments |
63,954 |
|
(109,126) |
|
Total Other Comprehensive Income (Loss) |
63,954 |
|
(109,126) |
|
|
|
|
|
|
Net Comprehensive Loss |
(14,705,253) |
|
(12,014,404) |
|
|
|
|
|
|
Net Loss Per Share – Basic and Diluted |
(0.56) |
|
(0.52) |
|
|
|
|
|
|
Weighted Average Shares Outstanding – Basic and Diluted |
26,389,580 |
|
23,049,089 |
(The accompanying notes are an integral part of these consolidated financial statements)
F-4
VOLITIONRX LIMITED
Consolidated Statements of Cash Flows
(Expressed in United States Dollars)
|
|
For the year ended December 31, 2017 |
|
For the year ended December 31, 2016 |
|
|
$ |
|
$ |
|
Operating Activities |
|
|
|
|
|
|
|
|
|
Net loss |
(14,769,207) |
|
(11,905,278) |
|
|
|
|
|
|
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
Depreciation and amortization |
528,166 |
|
309,406 |
|
Loss on disposal of property & equipment |
38,941 |
|
3,668 |
|
Stock-based compensation |
2,435,088 |
|
1,678,748 |
|
Warrants issued for services |
54,510 |
|
164,173 |
|
|
|
|
|
|
Changes in operating assets and liabilities: |
|
|
|
|
Prepaid expenses |
(82,733) |
|
(13,168) |
|
Deferred grant income |
(50,855) |
|
(170,343) |
|
Other current assets |
(7,486) |
|
(22,859) |
|
Accounts payable and accrued liabilities |
(339,662) |
|
1,090,942 |
|
Net Cash Used in Operating Activities |
(12,193,238) |
|
(8,864,711) |
|
|
|
|
|
|
Investing Activities |
|
|
|
|
Purchases of property and equipment |
(1,425,215) |
|
(415,091) |
|
|
|
|
|
|
Net Cash Used in Investing Activities |
(1,425,215) |
|
(415,091) |
|
|
|
|
|
|
Financing Activities |
|
|
|
|
Net proceeds from issuance of common shares |
998,413 |
|
25,302,274 |
|
Proceeds from debt payable |
1,201,980 |
|
474,769 |
|
Grants repaid |
(45,422) |
|
(36,135) |
|
Loans repaid |
(52,420) |
|
- |
|
Payments on capital lease obligations |
(135,597) |
|
(552,534) |
|
Net Cash Provided by Financing Activities |
1,966,954 |
|
25,188,374 |
|
|
|
|
|
|
Effect of foreign exchange on cash and cash equivalents |
89,028 |
|
(145,844) |
|
|
|
|
|
|
Net change in cash and cash equivalents |
(11,562,471) |
|
15,762,728 |
|
|
|
|
|
|
Cash and cash equivalents – Beginning of Period |
21,678,734 |
|
5,916,006 |
|
|
|
|
|
|
Cash and cash equivalents – End of Period |
10,116,263 |
|
21,678,734 |
|
|
|
|
|
(The accompanying notes are an integral part of these consolidated financial statements)
F-5
VOLITIONRX LIMITED
Consolidated Statements of Cash Flows (Continued)
(Expressed in United States Dollars)
|
Supplemental Disclosures of Cash Flow Information: |
|
|
|
|
|
|
|
|
|
|
|
Interest paid |
$ |
73,133 |
$ |
20,378 |
|
Income tax paid |
|
- |
|
- |
|
|
|
|
|
|
|
Non- Cash Financing Activities: |
|
|
|
|
|
|
|
|
|
|
|
Common stock issued on cashless exercises of stock options |
|
1 |
|
54 |
|
Capital lease obligation for equipment purchases |
$ |
- |
$ |
1,008,826 |
F-6
VOLITIONRX LIMITED
Consolidated Statement of Stockholders’ Equity
For the Years Ended December 31, 2017 and 2016
(Expressed in United States Dollars)
|
|
Common Stock |
Additional Paid-in Capital $ |
Accumulated Other Comprehensive Loss $ |
Accumulated Deficit $ |
Total $ |
|
|
|
Shares |
Amount $ |
||||
|
Balance, December 31, 2015 |
18,763,272 |
18,763 |
35,149,420 |
(84,171) |
(29,039,693) |
6,044,319 |
|
|
|
|
|
|
|
|
|
Common stock issued for cash, net of issuance costs |
7,309,120 |
7,309 |
25,294,965 |
- |
- |
25,302,274 |
|
|
|
|
|
|
|
|
|
Common stock issued for cashless exercise of stock options |
53,657 |
54 |
(54) |
- |
- |
- |
|
|
|
|
|
|
|
|
|
Employee stock options granted for services |
- |
- |
1,678,748 |
- |
- |
1,678,748 |
|
|
|
|
|
|
|
|
|
Warrants granted for services |
- |
- |
164,173 |
- |
- |
164,173 |
|
|
|
|
|
|
|
|
|
Other comprehensive loss |
- |
- |
- |
(109,126) |
- |
(109,126) |
|
|
|
|
|
|
|
|
|
Net loss for the year |
- |
- |
- |
- |
(11,905,278) |
(11,905,278) |
|
|
|
|
|
|
|
|
|
Balance, December 31, 2016 |
26,126,049 |
26,126 |
62,287,252 |
(193,297) |
(40,944,971) |
21,175,110 |
|
|
|
|
|
|
|
|
|
Common stock issued for cash |
392,651 |
392 |
998,021 |
- |
- |
998,413 |
|
|
|
|
|
|
|
|
|
Common stock issued for cashless exercise of stock options |
694 |
1 |
(1) |
- |
- |
- |
|
|
|
|
|
|
|
|
|
Employee stock options granted for services |
- |
- |
2,435,088 |
- |
- |
2,435,088 |
|
|
|
|
|
|
|
|
|
Warrants granted for services |
- |
- |
54,510 |
- |
- |
54,510 |
|
|
|
|
|
|
|
|
|
Other comprehensive income |
- |
- |
- |
63,954 |
- |
63,954 |
|
|
|
|
|
|
|
|
|
Net loss for the year |
- |
- |
- |
- |
(14,769,207) |
(14,769,207) |
|
|
|
|
|
|
|
|
|
Balance, December 31, 2017 |
26,519,394 |
26,519 |
65,774,870 |
(129,343) |
(55,714,178) |
9,957,868 |
(The accompanying notes are an integral part of these consolidated financial statements)
F-7
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For the Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 1 – Nature of Operations
The Company was incorporated under the laws of the State of Delaware on September 24, 1998. On September 22, 2011, the Company filed a Certificate for Renewal and Revival of Charter with Secretary of State of Delaware. Pursuant to Section 312(1) of the Delaware General Corporation Law, the Company was revived under the new name of “VolitionRX Limited”. The name change to VolitionRX Limited was approved by FINRA on October 7, 2011 and became effective on October 11, 2011.
On October 6, 2011, the Company entered into a share exchange agreement with Singapore Volition Pte. Limited., a Singapore corporation (“Singapore Volition”), and the shareholders of Singapore Volition, which was incorporated on August 5, 2010. Pursuant to the terms of the share exchange agreement, the former shareholders of Singapore Volition held 85% of the issued and outstanding common shares of the Company. The issuance was deemed to be a reverse acquisition for accounting purposes. Singapore Volition, the acquired entity, is regarded as the predecessor entity as of October 6, 2011. The number of shares outstanding and per share amounts has been restated to recognize the recapitalization.
The Company’s principal business objective through its subsidiaries is to develop and bring to market simple, easy to use blood-based cancer tests to accurately diagnose a range of cancers. The tests are based on the science of Nucleosomics, which is the practice of identifying and measuring nucleosomes in the bloodstream or other bodily fluid – an indication that disease is present. The Company has one wholly-owned subsidiary, Singapore Volition, which it acquired through a share exchange entered into on October 6, 2011. Singapore Volition has two wholly owned subsidiaries, Belgian Volition SPRL, a Belgium private limited liability company (“Belgian Volition”), which it acquired as of September 22, 2010, and Hypergenomics Pte. Limited (“Hypergenomics”), which it formed as of March 7, 2011. Belgian Volition, has two wholly owned subsidiaries, Volition Diagnostics UK Limited, which it formed as of November 13, 2015 and Volition America, Inc., which it formed as of February 3, 2017. Following the acquisition of Singapore Volition, the Company’s fiscal year end was changed from August 31 to December 31.
Note 2 - Going Concern
The Company's financial statements are prepared using generally accepted accounting principles in the United States of America (“U.S. GAAP”) applicable to a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business. The Company has incurred losses since inception of $55.7 million, has negative cash flows from operations, and currently no revenues, which creates substantial doubt about its ability to continue as a going concern.
The future of the Company as an operating business will depend on its ability to obtain sufficient capital contributions, financing and/or generate revenues as may be required to sustain its operations. Management plans to address the above as needed by; (a) securing additional grant funds; (b) obtaining additional equity or debt financing; (c) grants of licensing rights to third parties in exchange for specified up-front and/or back end payments; and (d) developing and commercializing its products on an accelerated timeline. Management continues to exercise tight cost controls to conserve cash.
The ability of the Company to continue as a going concern is dependent upon its accomplishment of the plans described in the preceding paragraph and eventually to attain profitable operations. The accompanying financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern. If the Company is unable to obtain adequate capital, it could be forced to cease operations.
F-8
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For the Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies
Basis of Presentation
The financial statements of the Company have been prepared in accordance with U.S. GAAP and are expressed in United States dollars. The Company’s fiscal year end is December 31.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company also regularly evaluates estimates and assumptions related to deferred income tax asset valuation allowances, impairment analysis of intangible assets and valuations of equity-based payments.
The Company bases its estimates and assumptions on current facts, historical experiences and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company’s estimates. To the extent there are material differences between the estimates and the actual results, future results of operations could be affected.
Principles of Consolidation
The accompanying consolidated financial statements for the year ended December 31, 2017 include the accounts of the Company and its wholly-owned subsidiaries, Volition America, Singapore Volition, Belgian Volition, Hypergenomics and Volition Diagnostics UK Limited. All significant intercompany balances and transactions have been eliminated in consolidation.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with a maturity of three months or less at the time of issuance to be cash equivalents. At December 31, 2017 and December 31, 2016, the Company had $10,116,263 and $21,678,734, respectively, in cash and cash equivalents. At December 31, 2017 and December 31, 2016, the Company had approximately $7,369,647 and $17,154,377, respectively, in its domestic accounts in excess of Federal Deposit Insurance Corporation insured limits. At December 31, 2017 and December 31, 2016, the Company had approximately $1,921,115 and $2,401,894, respectively, in its foreign accounts in excess of the Belgian Deposit Guarantee insured limits. At December 31, 2017 and December 31, 2016, the Company had approximately $161,189 and $1,719,937, respectively, in its foreign accounts in excess of the Singapore Deposit Insurance Scheme. At December 31, 2017 and December 31, 2016, the Company had $184,234 and $nil, respectively, in its foreign accounts in excess of the UK Deposit Protection Scheme.
Basic and Diluted Net Loss Per Share
The Company computes net loss per share in accordance with ASC 260, Earnings Per Share, which requires presentation of both basic and diluted earnings per share (EPS) on the face of the income statement. Basic EPS is computed by dividing net loss available to common shareholders (numerator) by the weighted average number of shares outstanding (denominator) during the period. Diluted EPS gives effect to all dilutive potential common shares outstanding during the period using the treasury stock method and convertible preferred stock using the if-converted method. In computing diluted EPS, the average stock price for the period is used in determining the number of shares assumed to be purchased from the exercise of stock options or warrants. As of December 31, 2017, 2,357,380 dilutive warrants and options were excluded from the diluted EPS calculation as their effect is anti-dilutive. As of December 31, 2016, 2,548,666 dilutive warrants and options were excluded from the diluted EPS calculation as their effect is anti-dilutive.
Foreign Currency Translation
The Company has functional currencies in the Euro, the United States dollar and British Pounds Sterling and its reporting currency is the United States dollar. Management has adopted ASC 830-20, “Foreign Currency Matters – Foreign Currency Transactions”. All assets and liabilities denominated in foreign currencies are translated using the exchange rate prevailing at the balance sheet date. For revenues and expenses, the weighted average exchange rate for the period is used. Gains and losses arising on translation of foreign currency denominated transactions are included in Other Comprehensive Income (Loss).
F-9
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For the Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies (Continued)
Financial Instruments
Pursuant to ASC 820, Fair Value Measurements and Disclosures, an entity is required to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 820 prioritizes the inputs into three levels that may be used to measure fair value:
Level 1
Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2
Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the assets or liabilities such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3
Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
The Company’s financial instruments consist principally of cash, accounts receivable, accounts payable, accrued liabilities, notes payable, and amounts due to related parties. Pursuant to ASC 820, the fair value of cash is determined based on “Level 1” inputs, which consists of quoted prices in active markets for identical assets. The Company believes that the recorded values of all of our other financial instruments approximate their current fair values because of their nature and respective maturity dates or durations.
Income Taxes
Potential benefits of income tax losses are not recognized in the accounts until realization is more likely than not. The Company has adopted ASC 740, Accounting for Income Taxes as of its inception. Pursuant to ASC 740, the Company is required to compute tax asset benefits for net operating losses carried forward. The potential benefits of net operating losses have not been recognized in these financial statements because the Company cannot be assured it is more likely than not it will utilize the net operating losses carried forward in future years.
Other Comprehensive Loss
ASC 220, Other Comprehensive Loss, establishes standards for the reporting and display of other comprehensive loss and its components in the financial statements. At December 31, 2017, the Company had $129,374 of accumulated other comprehensive loss, relating to foreign currency translation.
Revenue Recognition
The Company recognizes revenue when all of the following have occurred (i) persuasive evidence of an arrangement exists, (ii) delivery has occurred or services have been rendered, (iii) the price is fixed or determinable and (iv) the ability to collect is reasonably assured.
Research and Development
In accordance with ASC 730, the Company follows the policy of expensing its research and development costs in the period in which they are incurred. The Company incurred research and development expenses of $8.9 million and $7.9 million during the years ended December 31, 2017 and 2016, respectively.
F-10
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For the Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies (Continued)
Impairment of Long-Lived Assets
In accordance with ASC 360, Property Plant and Equipment, the Company tests long-lived assets or asset groups for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. Circumstances which could trigger a review include, but are not limited to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; and current expectation that the asset will more likely than not be sold or disposed of significantly before the end of its estimated useful life. Recoverability is assessed based on the carrying amount of the asset and its fair value which is generally determined based on the sum of the undiscounted cash flows expected to result from the use and the eventual disposal of the asset, as well as specific appraisal in certain instances. An impairment loss is recognized when the carrying amount is not recoverable and exceeds fair value. Impairment losses of $nil and $nil were recognized during the years ended December 31, 2017 and December 31, 2016, respectively.
Stock-Based Compensation
The Company records stock-based compensation in accordance with ASC 718, Compensation – Stock Compensation and ASC 505-50, Equity-Based Payments to Non-Employees. All transactions in which goods or services are the consideration received for the issuance of equity instruments are accounted for based on the fair value of the consideration received or the fair value of the equity instrument issued, whichever is more reliably measurable. Equity instruments issued to employees and the cost of the services received as consideration are measured and recognized based on the fair value of the equity instruments issued and are recognized over the employees required service period, which is generally the vesting period.
Grants received
The Company receives funding from public bodies for a proportion of the costs of specific projects. Funds are received in line with claims submitted for the agreed expenditure. The Company recognizes grant income once claims submitted are approved and funds are received. General working capital funding received at the commencement of a project is treated as deferred income until it has been utilized for the expenditure claimed. Funding received that is repayable is shown as a liability.
Reclassification
Certain balances in previously issued financial statements have been reclassified to be consistent with the current period presentation.
Recent Accounting Pronouncements
The Company has implemented all new accounting pronouncements that are in effect. The Company does not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial position or results of operations.
F-11
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For the Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 4 - Property and Equipment
The Company’s property and equipment consist of the following amounts as of December 31, 2017 and 2016:
|
|
|
|
|
|
|
|
|
December 31, |
|
|
|
|
|
|
|
|
|
2017 |
|
|
|
|
|
|
|
Accumulated |
|
Net Carrying |
|
|
|
|
|
Cost |
|
Depreciation |
|
Value |
|
|
|
Useful Life |
|
$ |
|
$ |
|
$ |
|
Computer hardware and software |
|
3 years |
|
239,133 |
|
93,422 |
|
145,711 |
|
Laboratory equipment |
|
5 years |
|
1,575,354 |
|
653,636 |
|
921,718 |
|
Office furniture and equipment |
|
5 years |
|
207,208 |
|
54,479 |
|
152,729 |
|
Buildings |
|
30 years |
|
1,571,004 |
|
43,632 |
|
1,527,372 |
|
Building improvements |
|
5-15 years |
|
673,157 |
|
35,748 |
|
637,409 |
|
Land |
|
Not amortized |
|
95,843 |
|
- |
|
95,843 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
4,361,699 |
|
880,917 |
|
3,480,782 |
|
|
|
|
|
|
|
|
|
December 31, |
|
|
|
|
|
|
|
|
|
2016 |
|
|
|
|
|
|
|
Accumulated |
|
Net Carrying |
|
|
|
|
|
Cost |
|
Depreciation |
|
Value |
|
|
|
Useful Life |
|
$ |
|
$ |
|
$ |
|
Computer hardware and software |
|
3 years |
|
157,002 |
|
68,229 |
|
88,773 |
|
Laboratory equipment |
|
5 years |
|
892,485 |
|
334,837 |
|
557,648 |
|
Office furniture and equipment |
|
5 years |
|
32,932 |
|
23,361 |
|
9,571 |
|
Buildings |
|
30 years |
|
1,378,911 |
|
- |
|
1,378,911 |
|
Building improvements |
|
5-15 years |
|
- |
|
- |
|
- |
|
Land |
|
Not amortized |
|
84,124 |
|
- |
|
84,124 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
2,545,454 |
|
426,427 |
|
2,119,027 |
Effective, October 25, 2016, the Company entered into a Real Estate Capital Lease Agreement to purchase real property for a total sum of €1,120,000 Euros. See Note 10(c) for additional details of the building purchase and the capital lease.
The majority of capital expenditures in 2017 related to €0.7 million Euros for building improvements in our laboratory and €0.7 million Euros related to new research and development laboratory equipment.
During the years ended December 31, 2017 and 2016, the Company recognized $454,490 and $222,944 in depreciation expense respectively.
F-12
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For the Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 5 - Intangible Assets
The Company’s intangible assets consist of intellectual property and patents, mainly acquired in the acquisition of ValiBio SA. The patents and intellectual property are being amortized over the assets’ estimated useful lives, which range from 8 to 20 years.
|
|
|
|
|
|
|
December 31, |
|
|
|
|
|
|
|
2017 |
|
|
|
|
|
Accumulated |
|
Net Carrying |
|
|
|
Cost |
|
Amortization |
|
Value |
|
|
|
$ |
|
$ |
|
$ |
|
|
|
|
|
|
|
|
|
Patents |
|
1,213,314 |
|
636,917 |
|
576,397 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
December 31, |
|
|
|
|
|
|
|
2016 |
|
|
|
|
|
Accumulated |
|
Net Carrying |
|
|
|
Cost |
|
Amortization |
|
Value |
|
|
|
$ |
|
$ |
|
$ |
|
|
|
|
|
|
|
|
|
Patents |
|
1,085,133 |
|
482,940 |
|
602,193 |
|
|
|
|
|
|
|
|
During the years ended December 31, 2017 and 2016, the Company recognized $87,994 and $86,462, respectively, in amortization expense. No impairment losses were recognized during the years ended December 31, 2017 and December 31, 2016.
The Company amortizes the long-lived assets on a straight-line basis with terms of 8 to 20 years. The annual estimated amortization schedule over the next five years is as follows:
|
|
|
|
|
|
2018 |
|
$ |
92,610 |
|
2019 |
|
$ |
92,610 |
|
2020 |
|
$ |
92,610 |
|
2021 |
|
$ |
92,610 |
|
2022 |
|
$ |
92,610 |
|
Thereafter |
|
$ |
113,347 |
|
|
|
|
|
|
Total |
|
$ |
576,397 |
The Company periodically reviews its long-lived assets to ensure that their carrying value does not exceed their fair market value. The Company carried out such a review in accordance with ASC 360 as of December 31, 2017. The result of this review confirmed that the ongoing value of the patents was not impaired as of December 31, 2017.
F-13
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For the Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 6 - Related Party Transactions
The Company has an agreement with a related party for consultancy services for a Company subsidiary. See Note 10(b) for obligations under the agreement. The Company issued shares of common stock to related parties upon the exercise of warrants and stock options. See Note 7 for details regarding such issuances.
Note 7 - Common Stock
2017
During 2017, 32,500 warrants were exercised at a price of $2.20 per share, for net cash proceeds to the Company of $71,500. As a result, a total of 32,500 shares of common stock were issued.
During 2017, 47,000 warrants were exercised at a price of $2.40 per share, for net cash proceeds to the Company of $112,800. As a result, a total of 47,000 shares of common stock were issued.
During 2017, 313,151 warrants were exercised at a price of $2.60 per share, for net cash proceeds to the Company of $814,193. As a result, a total of 313,151 shares of common stock were issued.
During 2017, 4,166 stock options were exercised to purchase shares of common stock at $3.00 per share in a cashless exercise that resulted in the issuance of 694 shares of common stock.
2016
During 2016, 400,000 warrants were exercised at a price of $0.50 per share, for net cash proceeds to the Company of $200,000. As a result, a total of 400,000 shares of common stock were issued.
During 2016, 87,500 warrants were exercised at a price of $2.20 per share, for net cash proceeds to the Company of $192,500. As a result, a total of 87,500 shares of common stock were issued.
During 2016, 2,601 warrants were exercised at a price of $2.60 per share, for net cash proceeds to the Company of $6,763. As a result, a total of 2,601 shares of common stock were issued.
During 2016, 235,000 stock options were exercised to purchase shares of our common stock at $2.50 to $3.00 per share in cashless exercises that resulted in the issuance of 53,657 shares of common stock.
During 2016, the Company issued 4,334,615 shares of common stock at a price of $3.25 per share, for net cash proceeds of approximately $13.1 million. Additionally, $0.3 million was incurred on legal expenses and stock market listing fees, resulting in net cash proceeds to the Company of $12.8 million.
During 2016, the Company issued 2,250,000 shares of common stock at a price of $5.00 per share, for net cash proceeds of approximately $10.7 million. Additionally, $0.1 million was incurred on legal expenses and stock market listing fees, resulting in net cash proceeds to the Company of approximately $10.6 million.
During 2016, the Company issued 234,404 shares of common stock at a price of $5.00 per share, for net cash proceeds to the Company of approximately $1.1 million.
F-14
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For the Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 8 – Warrants and Options
a)Warrants
2017
The following table summarizes the changes in warrants outstanding of the Company during the year ended December 31, 2017:
|
|
|
Number of Warrants |
|
Weighted Average Exercise Price ($) |
|
Outstanding, December 31, 2016 |
|
2,162,638 |
|
2.40 |
|
Granted |
|
- |
|
- |
|
Exercised |
|
(392,651) |
|
2.38 |
|
Expired |
|
(38,307) |
|
2.40 |
|
Outstanding, December 31, 2017 |
|
1,731,680 |
|
2.36 |
|
|
|
|
|
|
|
Exercisable, December 31, 2017 |
|
1,606,680 |
|
2.35 |
During 2017, 392,651 warrants were exercised for net cash proceeds to the Company of $998,493. Refer to Note 7 for the details of the exercises.
During 2017, 38,307 warrants expired.
On February 14, 2017, as a result of a modification of a warrant agreement, the Company re-measured warrants held by an employee, to purchase 25,000 shares of common stock at an exercise price of $2.47 per share. These warrants vest on achievement of certain business objectives and expire 3 years from the date of vesting. The Company has calculated the estimated fair market value of these warrants using the Black-Scholes model and the following assumptions: term: 0.5 years, stock price: $4.52, exercise price: $2.47, 55.65% volatility, 0.66% risk free rate.
On August 22, 2017, the Company amended the expiry period of 24,000 warrants, originally granted on September 26, 2014. The expiration period was extended from three to four years for all 24,000 warrants, with their new expiration date being September 26, 2018. The Company recalculated the estimated fair market value of these warrants using the Black-Scholes model, but the result was deemed to be immaterially different to the original calculation and the financial statements were not adjusted.
On August 22, 2017, the Company amended the expiry period of 19,000 warrants, originally granted on November 17, 2014. The expiration period was extended from three to four years for all 19,000 warrants, with their new expiration date being November 17, 2018. The Company recalculated the estimated fair market value of these warrants using the Black-Scholes model, but the result was deemed to be immaterially different to the original calculation and the financial statements were not adjusted.
2016
During 2016, 490,101 warrants were exercised for net cash proceeds to the Company of $399,263. Refer to Note 7 for the details of the exercises.
On May 4, 2016, the Company amended the expiry period of warrants to purchase 341,458 shares, originally granted on May 11, 2012, with an exercise price of $2.60. The expiration period was extended from May 10, 2016 to May 10, 2017.
On July 11, 2016, the Company amended the expiry period of warrants to purchase 45,000 shares, originally granted on August 7, 2013, with an exercise price of $2.40. The expiration period was extended from August 7, 2016 to August 7, 2017.
On November 14, 2016, the Company granted warrants to purchase 40,000 shares of common stock at an exercise price of $4.53 per share. These warrants vested on the date of grant and expire four years from the date of vesting.
F-15
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For the Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 8 – Warrants and Options (Continued)
Below is a table summarizing the warrants issued and outstanding as of December 31, 2017, which have a weighted average exercise price of $2.36 per share and a weighted average remaining contractual life of 1.43 years.
|
Number Outstanding |
|
Number Exercisable |
|
Exercise Price ($) |
|
Weighted Average Remaining Contractual Life (Years) |
|
Proceeds to Company if Exercised ($) |
|
|
|
29,750 |
|
29,750 |
|
2.00 |
|
0.01 |
|
59,500 |
|
|
948,475 |
|
948,475 |
|
2.20 |
|
0.63 |
|
2,086,645 |
|
|
520,455 |
|
520,455 |
|
2.40 |
|
0.27 |
|
1,249,092 |
|
|
150,000 |
|
25,000 |
|
2.47 |
|
0.43 |
|
370,500 |
|
|
24,000 |
|
24,000 |
|
3.00 |
|
0.01 |
|
72,000 |
|
|
19,000 |
|
19,000 |
|
3.75 |
|
0.01 |
|
71,250 |
|
|
40,000 |
|
40,000 |
|
4.53 |
|
0.07 |
|
181,200 |
|
|
1,731,680 |
|
1,606,680 |
|
|
|
1.43 |
|
4,090,187 |
Warrant option expense of $54,510 and $164,173 was recorded in the years ended December 31, 2017 and December 31, 2016, respectively. Total remaining unrecognized compensation cost related to non-vested warrants is approximately $25,590 and is expected to be recognized over a period of 3.0 years. As of December 31, 2017, the total intrinsic value of warrants was $1,081,382.
F-16
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For the Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 8 – Warrants and Options (Continued)
b)Options
The Company currently has options outstanding under both its 2011 Equity Incentive Plan (the “2011 Plan”) (for option issuances prior to 2016) and its 2015 Stock Incentive Plan (as amended, the “2015 Plan”) (for option issuances commencing in 2016). Effective as of January 1, 2016, no additional awards were or may be made under the 2011 Plan.
The 2015 Plan was adopted by the Board of Directors on August 18, 2015 and approved by the stockholders at an annual meeting held on October 30, 2015. On August 5, 2016, the Board of Directors adopted an amendment to the 2015 Plan to increase the number of shares of common stock available for issuance under such Plan by 750,000 shares to an aggregate maximum of 1,750,000 shares, which amendment was approved by the stockholders at an annual meeting held on October 7, 2016. On June 13, 2017, the Board of Directors adopted a subsequent amendment to the 2015 Plan to increase the number of shares of common stock available for issuance under such Plan by 750,000 shares to an aggregate maximum of 2,500,000 shares, which amendment was approved by the stockholders at an annual meeting held on September 8, 2017. The 2015 Plan permits the grant of incentive stock options, non-statutory stock options, restricted stock awards, stock bonus awards, stock appreciation rights, restricted stock units and performance awards. The primary purpose of the 2015 Plan is to enhance the Company’s ability to attract and retain the services of qualified employees, officers, directors, consultants and other service providers upon whose judgment, initiative and efforts the successful conduct and development of the Company’s business largely depends, and to provide additional incentives to such persons or entities to devote their utmost effort and skill to the advancement and betterment of the Company, by providing them an opportunity to participate in the ownership of the Company that is tied to the Company’s performance, thereby giving them an interest in the success and increased value of the Company. The 2015 Plan is administered by the Compensation Committee comprised solely of members of the Board of Directors or by the Board of Directors as a whole.
The following table summarizes the changes in options outstanding of the Company during the year ended December 31, 2017:
|
|
|
Number of Options |
|
Weighted Average Exercise Price ($) |
|
Outstanding, December 31, 2016 |
|
2,384,300 |
|
3.75 |
|
Granted |
|
871,000 |
|
4.99 |
|
Exercised |
|
(4,166) |
|
3.00 |
|
Expired |
|
(312,000) |
|
4.03 |
|
Outstanding, December 31, 2017 |
|
2,939,134 |
|
4.09 |
|
|
|
|
|
|
|
Exercisable, December 31, 2017 |
|
2,068,134 |
|
3.71 |
2017
During 2017, 4,166 stock options were exercised to purchase shares of common stock at $3.00 per share in a cashless exercise that resulted in the issuance of 694 shares of common stock.
During 2017, stock options to purchase 312,000 shares of common stock expired unexercised.
On January 1, 2017, the Company granted options to purchase 50,000 shares. These options vest on January 1, 2018 and expire 5 years after the vesting date, with an exercise price of $4.80 per share. The Company has calculated the estimated fair market value of these options at $157,890, using the Black-Scholes model and the following assumptions: term 6 years, stock price $4.57, exercise price $4.80, 80.70% volatility, 2.26% risk free rate.
On February 13, 2017, the Company granted options to purchase 25,000 shares. These options vest on February 13, 2018 and expire 5 years after the vesting date, with an exercise price of $5.00 per share. The Company has calculated the estimated fair market value of these options at $76,773, using the Black-Scholes model and the following assumptions: term 6 years, stock price $4.52, exercise price $5.00, 80.17% volatility, 2.24% risk free rate.
F-17
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For the Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 8 – Warrants and Options (Continued)
On March 30, 2017, the Company granted stock options to purchase 686,000 shares of common stock. These options vest on March 30, 2018 and expire five years after their vesting date, with an exercise price of $5.00 per share. The Company has calculated the estimated fair market value of these options at $1,898,322, using the Black-Scholes model and the following assumptions: term of 6 years, stock price $4.18, exercise price $5.00, 79.41% volatility, 2.25% risk free rate.
On April 10, 2017, the Company granted stock options to purchase 100,000 shares of common stock. These options vest on April 10, 2018 and expire 5 years after the vesting date, with an exercise price of $5.00 per share. The Company has calculated the estimated fair market value of these options at $258,077, using the Black-Scholes model and the following assumptions: term 6 years, stock price $3.96, exercise price $5.00, 79.33% volatility, 2.18% risk free rate.
On July 13, 2017, the Company granted stock options to purchase 10,000 shares of common stock. These options vest on July 13, 2018 and expire 5 years after the vesting date, with an exercise price of $5.00 per share. The Company has calculated the estimated fair market value of these options at $19,068, using the Black-Scholes model and the following assumptions: term 6 years, stock price $3.15, exercise price $5.00, 78.41% volatility, 2.16% risk free rate.
On August 14, 2017, the Company amended the expiry period of stock options to purchase 37,000 shares of common stock, which options were originally granted on March 20, 2013 and amended on June 27, 2016. The expiration period was extended from four to six years, with the outside expiration date of March 20, 2022, after vesting for all 37,000 stock options. The Company recalculated the estimated fair market value of these options using the Black-Scholes model, but the result was deemed to be immaterially different to the original calculation and the financial statements were not adjusted.
On August 14, 2017, the Company amended the expiry period of stock options to purchase 16,300 shares of common stock, which options were originally granted on September 2, 2013 and amended on June 27, 2016. The expiration period was extended from four to six years, with the outside expiration date of September 2, 2022, after vesting for all 16,300 stock options. The Company recalculated the estimated fair market value of these options using the Black-Scholes model, but the result was deemed to be immaterially different to the original calculation and the financial statements were not adjusted.
2016
During 2016, stock options to purchase 36,000 shares of common stock expired unexercised.
During 2016, 235,000 stock options were exercised to purchase shares of our common stock at $2.50 to $3.00 per share in cashless exercises that resulted in the issuance of 53,657 shares of common stock.
On April 15, 2016, the Company granted options to purchase 775,000 shares, at an exercise price of $4.00 per share. These options vest in full twelve months from the date of grant and expire five years from the date of vesting.
On June 23, 2016, the Company granted options to purchase 15,000 shares, at an exercise price of $4.00 per share. The options will vest in full twelve months from the date of grant and will expire five years from the date of vesting.
F-18
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For the Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 8 – Warrants and Options (Continued)
On June 27, 2016, the Company amended the expiry period of 37,000 options, originally granted pursuant to Stock Option Agreements dated March 20, 2013, with an exercise price of $2.35 to $4.35 per share. The expiration period was extended from three to four years from the date of vesting for all 37,000 stock options.
On June 27, 2016, the Company amended the expiry period of 16,300 options, originally granted on pursuant to Stock Option Agreements dated September 2, 2013, with an exercise price of $2.35 to $4.35 per share. The expiration period was extended from three to four years from the date of vesting for all 16,300 stock options.
On September 13, 2016, the Company granted options to purchase 25,000 shares, at an exercise price of $4.65 per share. The options will vest in full twelve months from the date of grant and will expire five years from the date of vesting.
On November 11, 2016, the Company granted options to purchase 10,000 shares, at an exercise price of $5.00 per share. These options vest immediately and expire six years from the date of vesting.
Below is a table summarizing the options issued and outstanding as of December 31, 2017, all of which were issued pursuant to the 2011 Plan (for option issuances prior to 2016) or the 2015 Plan (for option issuances commencing in 2016) which have a weighted average exercise price of $4.09 per share and a weighted average remaining contractual life of 3.43 years.
|
|
Number Outstanding |
|
Number Exercisable |
|
Exercise Price ($) |
|
Weighted Average Remaining Contractual Life (Years) |
|
Proceeds to Company if Exercised ($) |
|
|
17,766 |
|
17,766 |
|
2.35 |
|
0.01 |
|
41,750 |
|
|
322,500 |
|
322,500 |
|
2.50 |
|
0.12 |
|
806,250 |
|
|
326,667 |
|
326,667 |
|
3.00 |
|
0.23 |
|
980,001 |
|
|
17,767 |
|
17,767 |
|
3.35 |
|
0.02 |
|
59,519 |
|
|
20,000 |
|
20,000 |
|
3.80 |
|
0.01 |
|
76,000 |
|
|
1,115,333 |
|
1,115,333 |
|
4.00 |
|
1.38 |
|
4,461,332 |
|
|
17,767 |
|
17,767 |
|
4.35 |
|
0.02 |
|
77,286 |
|
|
50,000 |
|
- |
|
4.80 |
|
0.09 |
|
240,000 |
|
|
1,041,334 |
|
220,334 |
|
5.00 |
|
1.53 |
|
5,206,670 |
|
|
10,000 |
|
10,000 |
|
6.31 |
|
0.02 |
|
63,100 |
|
|
2,939,134 |
|
2,068,134 |
|
|
|
3.43 |
|
12,011,908 |
Stock option expense of $2,435,088 and $1,678,748 was recorded in the years ended December 31, 2017 and December 31, 2016, respectively. Total remaining unrecognized compensation cost related to non-vested stock options is approximately $553,407 and is expected to be recognized over a period of 0.5 year. As of December 31, 2017, the total intrinsic value of stock options was $152,382.
F-19
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For the Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 9 - Income Taxes
The Company has estimated net operating losses for the years ended December 31, 2017 and 2016 of $12.3 million and $11.0 million, respectively, available to offset taxable income in future years.
The significant components of deferred income taxes and assets as at December 31, 2017 are as follows:
|
Net deferred tax liability |
|
2017 $ |
|
2016 $ |
|
Excess of tax over book depreciation and amortization |
|
- |
|
- |
|
Prepaid expenses |
|
- |
|
- |
|
Allowance for doubtful accounts |
|
- |
|
- |
|
Accrued expenses |
|
1,154 |
|
- |
|
Stock-based compensation |
|
- |
|
- |
|
Net Operating Losses carry-forward |
|
11,156,839 |
|
8,806,016 |
|
Research and development tax credits |
|
- |
|
- |
|
Gross deferred tax assets |
|
11,157,993 |
|
8,806,016 |
|
Valuation allowance |
|
(11,157,993) |
|
(8,806,016) |
|
|
|
|
|
|
|
Net deferred tax asset |
|
- |
|
- |
|
Summary Rate Reconciliation |
|
2017 % |
|
|
|
Federal statutory rate |
|
35.0 |
|
|
|
State income taxes, net of federal benefit |
|
0.0 |
|
|
|
Permanent Differences |
|
0.1 |
|
|
|
Stock based compensation |
|
(5.8) |
|
|
|
Federal Research & Development Credits |
|
0.0 |
|
|
|
Foreign taxes |
|
(3.1) |
|
|
|
Federal Deferred Rate Decrease |
|
(10.3) |
|
|
|
Increase/(decrease) in valuation reserve |
|
(15.9) |
|
|
|
Total |
|
0.0 |
|
|
F-20
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 10 – Commitments and Contingencies
a)Walloon Region Grant
On March 16, 2010, the Company entered into an agreement with the Walloon Region government in Belgium wherein the Walloon Region would fund up to a maximum of €1,048,020 Euros to help the research endeavors of the Company in the area of colorectal cancer (“CRC”). The Company had received the entirety of these funds in respect of approved expenditures as of June 30, 2014. Under the terms of the agreement, the Company is due to repay €314,406 Euros of this amount by installments over the period from June 30, 2014 to June 30, 2023. The Company has recorded the balance of €733,614 Euros to other income in previous years as there is no obligation to repay this amount. In the event that the Company receives revenue from products or services as defined in the agreement, it is due to pay a 6% royalty on such revenue to the Walloon Region. The maximum amount payable to the Walloon Region, in respect of the aggregate of the amount repayable of €314,406 Euros and the 6% royalty on revenue, is twice the amount of funding received. As at December 31, 2017, $230,509 was outstanding to be repaid to the Walloon Region under this agreement.
b)Consulting Agreement
On May 11, 2016, Singapore Volition, upon the review and approval by the Company’s Compensation Committee, entered into a consultancy agreement with PB Commodities Pte. Ltd (“PB Commodities”), for the services of Cameron Reynolds (the “2016 Reynolds Consulting Agreement”). Under the terms of the 2016 Reynolds Consulting Agreement, PB Commodities shall receive $25,925 per month for the services provided to Singapore Volition by Mr. Reynolds on its behalf. The 2016 Reynolds Consulting Agreement replaced and terminated the existing consultancy agreement for the provision of office space, office support staff, and consultancy services between Singapore Volition and PB Commodities dated August 6, 2010, as amended. The 2016 Reynolds Consulting Agreement was terminated on March 31, 2017 in connection with Mr. Reynolds entering into an Employment Agreement with Volition Diagnostics UK Limited, effective April 1, 2017.
c)Lease Obligations Payable
The Company leases three Tecan machines (automated liquid handling robots) under a lease classified as a capital lease. The total cost of this leased laboratory equipment is €571,506 Euros. The leased equipment is amortized on a straight-line basis over five years. Total accumulated depreciation related to the leased equipment is $346,866 for the year ended December 31, 2017 and $183,296 for the year ended December 31, 2016.
On October 4, 2016, and effective on October 25, 2016, Belgian Volition entered into a Real Estate Capital Lease Agreement (the “Capital Lease Agreement”) with ING Asset Finance Belgium S.A. (“ING”). The Capital Lease Agreement became a contractual obligation of Belgian Volition upon the execution of the Deed of Sale to acquire the Company’s new research and development facility described below. Pursuant to the Capital Lease Agreement, ING paid €1.12 million Euros in return for Belgian Volition granting to ING a right of emphyteusis (a form of leasehold) on the property located in the Belgian Créalys zoning at 5032 Isnes-Spy, Rue Phocas Lejeune 22, Gembloux cadastre, 8th division, Section B, n 55 (the “Property”) for a period of 27 years, extendable to the authorized maximum legal term of 99 years. In addition, the Capital Lease Agreement provides that ING shall grant Belgian Volition a 15-year lease over the Property with an option for Belgian Volition to purchase the Property outright upon payment of €33,600 Euros at the end of the lease. The Capital Lease Agreement provides that Belgian Volition shall make the first lease payment of €440,000 Euros following the execution of the Capital Lease Agreement, and then quarterly lease payments of approximately €13,450 Euros, based on a fixed rate of 2.62% for the term of the lease. On October 25, 2016, Belgian Volition acquired the Property by entering into a Deed of Sale to the Sale Agreement with Gerard Dekoninck S.A. The purchase price for the Property consisted of €1.2 million Euros exclusive of any closing costs (the “Purchase Price”). The Purchase Price was funded by Belgian Volition with cash on hand and the monies received under the Capital Lease Agreement. The Company occupied the Property commencing in March 2017. Total depreciation charged to the income statement, related to the leased building was $43,633 for the year ended December 31, 2017 and $nil for the year ended December 31, 2016.
F-21
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 10 – Commitments and Contingencies (Continued)
The following is a schedule showing the future minimum lease payments under capital leases by years and the present value of the minimum payments as of December 31, 2017.
|
2018 |
$ |
163,100 |
|
2019 |
$ |
163,100 |
|
2020 |
$ |
112,141 |
|
2021 |
$ |
64,439 |
|
2022 |
$ |
64,438 |
|
Greater than 5 years |
$ |
604,092 |
|
Total minimum lease payments |
$ |
1,171,310 |
|
Less: Amount representing interest |
$ |
(157,542) |
|
|
|
|
|
Present value of minimum lease payments |
$ |
1,013,768 |
The Company also leases premises, facilities and motor vehicles under operating leases with terms ranging from 12 months to 36 months. The annual non-cancelable operating lease payments on these leases are as follows:
|
2018 |
$ |
214,152 |
|
2019 |
$ |
64,249 |
|
2020 |
$ |
51,022 |
|
2021 |
$ |
13,982 |
|
|
|
|
|
Total |
$ |
343,405 |
d)Hvidovre Hospital, Denmark Agreements
On November 2, 2016, the Company entered into a clinical research agreement with Hvidovre Hospital, University of Copenhagen in Denmark, relating to a program of samples testing associated with CRC and other diseases. This first phase of the agreement will expire on September 30, 2018 and the Company may participate in additional phases upon its election (and payment of required amounts). Total payments (inclusive of local taxes) to be made by the Company under the agreement for the first phase are DKR 15,000,000. As of December 31, 2017, approximately $1.03 million is still to be paid by the Company for the first phase by September 2018.
e)Munich University Agreement
On August 16, 2016, the Company entered into a collaborative research agreement with Munich University, Germany, relating to a program of samples testing. The agreement is for a period of three years from August 1, 2017 to July 31, 2020. The total payments payable by the Company in accordance with the agreement are €360,000 Euros. As of December 31, 2017, approximately $431,294 is still to be paid by the Company under this agreement.
f)Long Term Debt: Preface S.A. Loan Agreement
On September 16, 2016, Belgian Volition entered into an unsecured loan agreement with Namur Invest or Preface S.A. for the amount of €440,000 Euros (the “Loan Agreement”). The proceeds from the Loan Agreement were received by Belgian Volition on October 20, 2016. The Loan Agreement provides for an approximate 7-year term, a fixed interest rate at 4.85%, and interest only payments between the receipt of proceeds and June 30, 2017. The proceeds from this Loan Agreement were used for the first payment on the Real Estate Capital Lease Agreement. See Note 10(c) for additional details. As of December 31 2017, the balance payable in principal and interest was $492,212 and $76,072, respectively.
On May 2, 2017, Belgian Volition entered into an additional unsecured loan agreement with Namur Invest or Preface S.A. for the amount of €350,000 Euros (the “May 2017 Loan Agreement”). The May 2017 Loan Agreement provides for an approximate 3.5-year repayment term, a fixed interest rate at 4.00% and interest only payments between the receipt of proceeds and December 31, 2017. Thereafter, the May 2017 Loan Agreement requires monthly payments of €8,944 Euros. The proceeds from the May 2017 Loan Agreement will be used to fund a pathway study for– the Nu.Q Colorectal Cancer Screening Triage Test and other diagnostic products. As of December 31, 2017, the balance payable in principal and interest was $419,314 and $30,734, respectively.
F-22
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 10 – Commitments and Contingencies (Continued)
g) Long Term Debt: ING Loan Agreement
On October 25, 2016, Belgian Volition entered into a secured loan agreement with ING for an amount up to €270,000 Euros (the “Supplemental Loan”). The Supplemental Loan provides for a 15-year term commencing on March 31, 2017, a fixed interest rate at 2.96%, and quarterly repayments of €5,536 Euros, commencing on April 28, 2017. The maximum amount of the loan facility had been drawn down by Belgian Volition by the loan commencement date of March 31, 2017 and interest only payments were made from the initial draw down of the loan until September 30, 2017. The proceeds of the Supplemental Loan were used to finance the construction of a laboratory in the new research and development facility. See Note 10(c) for additional details. As of December 31, 2017, the balance payable in principal and interest was $306,030 and $65,405, respectively.
h)Clinical Study Agreement with the University of Michigan
On July 17, 2017, Volition America entered into a Clinical Study Agreement with the Regents of the University of Michigan (the “University of Michigan”), with regards to Volition America’s participation, with the University of Michigan and the National Cancer Institute Early Detection Research Network (“EDRN”), in a clinical study (the “University of Michigan Clinical Study Agreement”) involving approximately 13,500 samples. The enrollment period and sample collection is anticipated to take up to 3 years to complete. The total maximum payment due by Volition America in accordance with the agreement is $3 million spread over 12 equal quarterly installments of $250,000. As of December 31, 2017, $500,000 of costs have been incurred. The foregoing description of the University of Michigan Clinical Study Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.33.
i) National University Hospital Singapore Agreement
On July 15, 2017, the Company entered into a research collaboration with the National University of Singapore for cohorts in development of a CRC blood test. The agreement is for a period of two years from July 2017 to July 2019. The total payments payable by the Company in accordance with the agreement are $48,000. For the period ended December 2017, $38,400 has been paid and $9,600 is still payable by the Company under this agreement.
j)Straight Loan: ING Loan Agreement
On August 28, 2017, Belgian Volition received prefunding of €200,000 Euros from ING, pursuant to a loan agreement (the “Straight Loan Agreement”) entered into on December 13, 2016 and repayable upon receipt of grants for investment in Créalys business park from the Walloon Region. The term of the Straight Loan Agreement is until July 2018, on a rolling monthly basis at an interest rate of the Euribor rate + 2%. The proceeds of the Straight Loan Agreement were used to finance the investment in the Créalys business park. As of December 31, 2017, the balance payable in principal and interest was $239,608 and $9,584 respectively.
k)Long Term Debt: SOFINEX Loan Agreement
On September 20, 2017, VolitionRX and Belgian Volition entered into an unsecured loan agreement with SOFINEX, a Belgian public organization focused on the internationalization of Walloon companies, for an amount up to €1,000,000 Euros with draws of up to €250,000 Euros every six months through June 2019. The loan agreement provides for a 7-year repayment term, with a grace period for principal payments until December 31, 2019, and a fixed interest rate of 4.5%. As of December 31, 2017, €250,000 Euros cash has been drawn down under this agreement. As of December 31, 2017, the balance payable in principal and interest was $299,510 and $0 respectively.
l)Legal Proceedings
There are no legal proceedings which the Company believes will have a material adverse effect on its financial position.
F-23
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements (Continued)
For Years Ended December 31, 2017 and 2016
($ expressed in United States Dollars)
Note 11 - Subsequent Events
On January 23, 2018, the Company granted stock options to purchase 780,000 shares of common stock. These options vest on January 23, 2019 and expire 5 years after the vesting date, with an exercise price of $4.00 per share.
On January 31, 2018, stock options to purchase 10,000 shares of common stock expired unexercised.
On February 5, 2018, 26,400 warrants were exercised at $2.00 per share in a cashless exercise that resulted in the issuance of 11,399 shares of common stock.
END NOTES TO FINANCIALS
F-24
ITEM 9.CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE
None.
ITEM 9A.CONTROLS AND PROCEDURES
Disclosure Controls and Procedures
Disclosure controls and procedures are controls and procedures that are designed to ensure that information required to be disclosed in our reports filed under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by our company in the reports that it files or submits under the Exchange Act is accumulated and communicated to our management, including our Principal Executive and Principal Financial Officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Our management carried out an evaluation under the supervision and with the participation of our Principal Executive Officer and Principal Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures pursuant to Rules 13a-15(e) and 15d-15(e) under the Exchange Act. Based upon that evaluation, our Principal Executive Officer and Principal Financial Officer have concluded that, as of December 31, 2017, our disclosure controls and procedures were not effective because of material weakness in our internal control over financial reporting.
Management’s Report on Internal Control over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rule 13a-15(f). The Company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles generally accepted in the United States of America.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of management, including the Principal Executive Officer and Principal Financial Officer, the Company conducted an evaluation of the effectiveness of the Company’s internal control over financial reporting as of December 31, 2017, using the criteria established in “Internal Control - Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”).
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis. In its assessment of the effectiveness of internal control over financial reporting as of December 31, 2017, the Company determined that there were control deficiencies that constituted material weaknesses, as described below:
1.the Company did not maintain adequate segregation of duties in some areas of Finance; and
2.the Company did not maintain sufficient oversight in the areas of IT and Human Resources, where certain processes may affect the internal controls over financial reporting.
Accordingly, the Company concluded that these control deficiencies resulted in a possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis by the Company’s internal controls.
As a result of the material weaknesses described above, management has concluded that the Company did not maintain effective internal control over financial reporting as of December 31, 2017, based on criteria established in Internal Control—Integrated Framework issued by COSO.
25
Changes in Internal Control over Financial Reporting
The Audit Committee of the Board of Directors meets regularly with our financial management and counsel, and with the independent registered public accounting firm engaged by us. Internal accounting controls and the quality of financial reporting are discussed during these meetings. The Audit Committee has discussed with the independent registered public accounting firm matters required to be discussed by the auditing standards adopted or established by the Public Company Accounting Oversight Board. In addition, the Audit Committee and the independent registered public accounting firm have discussed the independent registered public accounting firm’s independence from the Company and its management, including the matters in the written disclosures required by Public Company Accounting Oversight Board Rule 3526 “Communicating with Audit Committees Concerning Independence”.
As of December 31, 2017, we did not maintain sufficient internal controls over financial reporting:
due to a lack of adequate segregation of duties in some areas of Finance; and
due to a lack of sufficient oversight in the areas of Information Technology (IT) and Human Resources, where certain processes may affect the internal controls over financial reporting.
We have developed, and are currently implementing, a remediation plan for these material weaknesses.
There have been no changes in our internal control over financial reporting during the fiscal fourth quarter of the year ended December 31, 2017 that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting.
The Company is not required by current SEC rules to include, and does not include, an auditor’s attestation report. Consequently, the Company’s registered public accounting firm has not attested to management’s reports on the Company’s internal control over financial reporting.
Continuing Remediation Efforts to address deficiencies in Company’s Internal Control over Financial Reporting
Once the Company is engaged in stable business operations and has sufficient personnel and resources available, then our Board of Directors, in particular and in connection with the aforementioned deficiencies, will establish the following remediation measures:
Additional Finance resources will be recruited to resolve the segregation of duties control weaknesses noted above.
Internal audit resources will be contracted to review and advise on control weaknesses across the organization.
Specialist resources in IT and Human Resources have been recruited to recommend and implement relevant policy and processes to strengthen IT and Human Resources internal controls associated with financial reporting.
ITEM 9B.OTHER INFORMATION
None.
26
PART III
ITEM 10. DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE
Identification of Directors
The following table sets forth the names and ages of the Company’s Directors as of December 31, 2017.
|
Name |
|
Age |
|
Position with the Company |
|
Officer/Director Since |
|
Cameron Reynolds |
|
46 |
|
President |
|
October 6, 2011 |
|
|
|
|
|
Chief Executive Officer |
|
October 6, 2011 |
|
|
|
|
|
Director |
|
October 6, 2011 |
|
Dr. Martin Faulkes |
|
73 |
|
Executive Chairman |
|
October 6, 2011 |
|
|
|
|
|
Director |
|
October 6, 2011 |
|
Guy Innes(1) (2) (3) |
|
61 |
|
Director |
|
October 6, 2011 |
|
Dr. Alan Colman(1) |
|
69 |
|
Director |
|
October 6, 2011 |
|
Dr. Habib Skaff(1) (2) (3) |
|
40 |
|
Director |
|
June 1, 2014 |
|
Dr. Edward Futcher(1) (2) (3) |
|
63 |
|
Director |
|
June 23, 2016 |
(1)Member of the Audit Committee
(2)Member of the Compensation Committee
(3)Member of the Nominations and Governance Committee
Term of Office
Each Director serves for a term of one year and until his or her successor is elected at the Annual Stockholders Meeting and is qualified, subject to removal by the stockholders.
Background and Business Experience of Directors
The business experience during the past five years of the directors is as follows:
CAMERON REYNOLDS serves as our President, Chief Executive Officer and Director. Prior to completion of the transactions under the Share Exchange Agreement, he was Chief Executive Officer and Director of Singapore Volition, a position he held since August 5, 2010. He is also a Director of Belgian Volition since October 27, 2010, serving as Managing Director between January 18, 2012 and July 24, 2015, a Director and Chief Executive Officer of Hypergenomics since March 7, 2011, was appointed Director and Chief Executive Officer of Volition Diagnostics UK Limited, on November 13, 2015, and was appointed Director of Volition America, Inc. on February 3, 2017. Since February 2017, Mr. Reynolds has concurrently served as a non-executive director of Ucroo Inc. From 2004 until 2011, Mr. Reynolds founded and served as Managing Director and Director of Mining House, where he was responsible for identifying potential mining projects, coordinating the preliminary evaluations and securing the financing with a view to listing the companies on the AIM, the TSX and the U.S. OTC. Mr. Reynolds furthered his education between 2002 and 2003 as he undertook an MBA. From 1998 until 2001, Mr. Reynolds served as the commercialization director for Probio, Inc., a company that commercialized intellectual property in the animal biotechnology fields including transgenesis and cloning research from the University of Hawaii. Mr. Reynolds’ main responsibilities were managing all legal and contract issues with the University of Hawaii; implementing patenting strategy; managing all stockholder issues including the merger and its legal and contractual documentation; head office management; budgetary control; team building and recruitment. Furthermore, Mr. Reynolds held a junior management position in 1996 at Integrated Coffee Technologies, a genetically modified coffee company where he was responsible for business plan creation, office management, recruitment, and business development. Starting in 1994, Mr. Reynolds was working for Southern China Group, where as regional manager he set up operations in Hong Kong and Yunnan. Between 2005 and 2011, Mr. Reynolds held a number of board directorships including Atlantic Mining PLC; Carbon Mining PLC, Magellan Copper and Gold (Carbon Mining and MCG both became part of Solfotara Mining and Copper Development Corp.); KAL Energy Inc. (OTC: KALG), Iofina Natural Gas PLC (AIM: IOF); Canyon Copper Corp. (TSX-V: CNC, OTCBB: CNYC), and Hunter Bay Resources (TSX-V: HBY). The Board of Directors believes Mr. Reynolds brings to the Company strong experience in management, structuring and strategic planning of start-up companies based on his over 20 years of entrepreneurial executive experience in the mining and biotechnology sectors.
27
DR. MARTIN FAULKES serves as Executive Chairman of the Board of Directors. Prior to completion of the transactions under the Share Exchange Agreement, Dr. Faulkes served as a Director of Singapore Volition from August 18, 2010 to December 15, 2015 and as Executive Chairman of the Board of Directors of Singapore Volition from March 22, 2011 until December 15, 2015. Dr. Faulkes also served as a Director of Belgian Volition between August 10, 2011 and March 31, 2016. From 1998 until the present day, Dr. Faulkes has focused on charitable activities, as the founder and sole benefactor of The Dill Faulkes Educational Trust, a U.K. registered charity, where he is Chairman. He also sits on the board of the Cambridge 800th Anniversary Campaign in the U.K. Prior to Dr. Faulkes’ charitable activities he founded Triad Plc., a computer software development company that provides systems and consultants to the business community, where he was a Director from 1987 to 1998, and responsible for controlling the company financially. From 1985 to 1987, he became Managing Director of System Programming Ltd., a company that provides computer programming for systems in businesses such as airlines, utility companies, banks, and insurance companies, where he was responsible for all aspects of the business. Prior to System Programming Ltd., Dr. Faulkes served from 1979 to 1984 as founder, President and Chief Executive Officer for Logica Inc., a company providing bespoke software to all industries but mainly banks and communications companies. Dr. Faulkes was responsible for all aspects of the business, including sales, finance, recruitment, staff management and project control. Dr. Faulkes has over 30 years of entrepreneurial and managerial experience as the founder and Chief Executive Officer of several software companies within the United Kingdom and the United States. The Board of Directors believes that Dr. Faulkes is qualified to serve as a director of the Company based on his extensive experience in business development and management.
GUY INNES serves as a Director. Prior to completion of the transactions under the Share Exchange Agreement, Mr. Innes served as a Director of Singapore Volition, a position he held from August 18, 2010 to December 15, 2015. Mr. Innes has served as a non-executive Director on the board of companies such as Carbon Mining Plc. from 2007 to 2010, Magellan Copper & Gold Plc. from 2007 to 2010, and ProBio Inc. from 2000 to 2006. As a non-executive Director, Mr. Innes was responsible for the development of corporate strategy and the implementation of financial controls and risk management systems. Mr. Innes had a long career in banking and private equity, including advisory roles with Quartz Capital Partners Limited from 1997 to 2000, where Mr. Innes served as Head of Corporate Finance and was responsible for managing the corporate finance department and leading the transactions undertaken by Quartz including IPOs, private placements and mergers and acquisitions; Baring Private Equity Partners Limited in London and Singapore from 1995 to 1997, where he was involved in the setting up, recruiting of managers and capital raising for an Asian media and communications private equity fund; and Baring Brothers & Co. Limited in London and Paris from 1984 to 1995, where he was involved in executing and advising on national and international mergers and acquisitions, but also IPOs and capital raising. Mr. Innes is a Chartered Accountant and a member of the Institute of Chartered Accountants in England and Wales. Mr. Innes has extensive experience in financing and managing technology companies. Our Board of Directors believes Mr. Innes’ technical, financial and managerial background would be beneficial to our growth.
DR. ALAN COLMAN serves as a Director. Prior to completion of the transactions under the Share Exchange Agreement, Dr. Colman served as a Director of Singapore Volition from April 1, 2011 to December 15, 2015 and currently serves as Chairman of the Scientific Advisory Board of Singapore Volition, a position he has held since April 5, 2011. Dr. Colman received a BA (1971), MA (1975) and Ph.D. (1975) from Oxford University. Dr. Colman is currently a Visiting Scholar at the Harvard University Department of Stem Cell and Regenerative Biology. He also currently serves on the Scientific Advisory Board of Semma Therapeutics, Inc., a stem cell therapy company based in Cambridge, Massachusetts, USA, a position he has held since December 2014. From 2007 to 2013, Dr. Colman served as the Executive Director of the Singapore Stem Cell Consortium. Concurrently, Dr. Colman was Professor of Regenerative Medicine at King’s College, London, U.K., from 2008 to 2009. Prior to joining the A*STAR Singapore Stem Cell Consortium, Dr. Colman was Chief Scientific Officer and then Chief Executive Officer for the Singaporean human embryonic stem cell company, ES Cell International from 2002 to 2007. Dr. Colman was the research director at PPL Therapeutics in Edinburgh, U.K., from the late 1980s until 2002, where he was responsible for leading PPL’s research program strategy, also playing a role in PPL’s financing rounds, culminating in its listing on the London Stock Exchange in 1996. PPL attracted considerable media attention because of its participation in the technique of somatic nuclear transfer that led to the world’s first sheep cloned from an adult cell, Dolly, in 1996. Dr. Colman had a successful university career in the Universities of Oxford, Warwick, Birmingham (where he was Professor of Biochemistry) and London (as mentioned above). None of the above companies or organizations is a parent, subsidiary or other affiliate of the Company. Dr. Colman’s current interest is the development of human disease models using induced pluripotent stem cells. He has extensive experience in the molecular biology field where he has worked in the production of transgenic livestock, somatic nuclear transfer, and human disease models. The Board of Directors appointed Dr. Colman a Director of the Company and a member of the Scientific Advisory Board based on his extensive experience in biochemistry, stem cell research and pathology.
28
DR. HABIB SKAFF serves as a Director. Prior to completion of the transactions under the Share Exchange Agreement, Dr. Skaff served as a Scientific Advisory Board Member of Singapore Volition between April 4, 2011 and May 31, 2014. Dr. Skaff currently serves as Managing Partner of Cedar Capital Holdings, LLC, where he heads operations as well as acquisitions of companies in fields varying from wound care to recycling. Dr. Skaff co-founded Intezyne Technologies in 2004 and served as its Chief Executive Officer until 2016. At Intezyne, Dr. Skaff was responsible for establishing and implementing strategic planning for the future, working closely with the Chief Scientific Officer to develop and implement Intezyne’s intellectual property strategy as well as establishing alliances with potential partners. As Chief Executive Officer, Dr. Skaff led Intezyne’s fundraising through debt and equity financing and worked closely with the Chief Financial Officer in this capacity. In addition, since 2001, Dr. Skaff has co-authored 11 peer-reviewed scientific papers and is a co-inventor on 34 pending or issued patents in the fields of chemistry, nanotechnology and biotechnology. Dr. Skaff works as a synthetic chemist specializing in the area of nanotechnology; his doctoral studies focused on the design of organic and polymeric ligands for the encapsulation of semiconductor nanoparticles and modification of the physical, optical, electronic, and assembly properties of the nanoparticles. Due to his extensive scholarly work and inventions in the fields of chemistry and biotechnology, the Board of Directors feels that Dr. Skaff is a valuable asset to the Company.
DR. EDWARD FUTCHER serves as a Director. Dr. Futcher holds a B.Sc. in Physics and a Ph.D. in Physics from the University of London and has extensive experience in engineering and management in high technology companies. Since 1997, Dr. Futcher has held non-executive directorships with a variety of private companies. He co-founded Azima, Inc. in 2003, a company that provides advanced machine diagnosis to large industrial facilities and, from 2003 to 2008, served as its Vice President of Engineering with responsibility for the engineering, information technology and customer support groups. Prior to that, from 1997 to 2003, Dr. Futcher served as Vice President of Technology of interWAVE Communications International, Ltd., a company providing GSM and CDMA cellular infrastructure equipment, where he was responsible for operational management of acquisitions and interim management of the worldwide research and development organization. From 1997 to 1999, Dr. Futcher also served as Vice President of Engineering of interWAVE Communications. From 1994 to 1997, Dr. Futcher was Director of Engineering at Tellabs, Inc., a telecommunications equipment supplier. The Board of Directors believes that Dr. Futcher is qualified to serve as a Director of the Company based on his extensive commercial and management experience in dynamic and fast growing companies.
Identification of Executive Officers
The following table sets forth the names and ages of the Company’s executive officers as of December 31, 2017.
|
Name |
|
Age |
|
Position with the Company |
|
Officer/Director Since |
|
Cameron Reynolds |
|
46 |
|
President |
|
October 6, 2011 |
|
|
|
|
|
Chief Executive Officer |
|
October 6, 2011 |
|
|
|
|
|
Director |
|
October 6, 2011 |
|
Dr. Martin Faulkes |
|
73 |
|
Executive Chairman |
|
October 6, 2011 |
|
|
|
|
|
Director |
|
October 6, 2011 |
|
David Vanston |
|
50 |
|
Chief Financial Officer |
|
April 10, 2017 |
|
|
|
|
|
Treasurer |
|
April 10, 2017 |
|
Rodney Rootsaert |
|
46 |
|
Secretary |
|
October 6, 2011 |
|
|
|
|
|
|
|
|
|
Dr. Jacob Micallef |
|
61 |
|
Chief Scientific Officer |
|
January 1, 2015 |
|
|
|
|
|
|
|
|
|
Dr. Jason Terrell |
|
37 |
|
Chief Medical Officer |
|
March 20, 2013 |
|
|
|
|
|
Head of U.S. Operations |
|
|
Term of Office
Each officer serves for such term as determined by their employment agreement as approved by the Board of Directors or Compensation Committee. See Item 11. Executive Compensation – Employment and Consulting Agreements.
29
Background and Business Experience of Executive Officers
The business experience during the past five years of the executive officers is as follows:
CAMERON REYNOLDS serves as our President and Chief Executive Officer and is a Director of the Company. Additional information regarding Mr. Reynolds is provided under “Item 10. — Directors, Executive Officers and Corporate Governance - Background and Business Experience of Directors” of this report.
DR. MARTIN FAULKES serves as our Executive Chairman of our Board of Directors. Additional information regarding Dr. Faulkes is provided under “Item 10. — Directors, Executive Officers and Corporate Governance - Background and Business Experience of Directors” of this report.
DAVID VANSTON serves as our Chief Financial Officer and Treasurer, a position he has held since April 2017. Mr. Vanston has over twenty years of financial management experience with a strong background as an international finance executive and a senior controller, including extensive experience in Sarbanes-Oxley compliance and implementation of successful change programs. Mr. Vanston was previously employed at Octo Telematics from 2016 to 2017, a high-growth technology company based in Boston, where he was the Senior Controller for the United States business. Prior to joining Octo Telematics, he was Vice President of Excorp Medical, Inc., an early-stage medical company in Minneapolis, from 2015 until 2016, and, from 2011 to 2015, he served as VP Finance/CFO in a consultant capacity to various listed/private companies including serving as Chief Financial Officer for GrowHow Ltd in 2013, in which Mr. Vanston managed and oversaw the accounting, finance, tax, treasury, financial planning and analysis. Mr. Vanston is a certified chartered accountant and holds an MBA from Warwick Business School. The Board of Directors believes that Mr. Vanston’s financial and accounting knowledge is a valuable asset to the Company.
RODNEY ROOTSAERT serves as our Secretary. Prior to the completion of the transactions under the Share Exchange Agreement, he was the Administration and Legal Officer of Singapore Volition, a position he held since August 6, 2010. Mr. Rootsaert became a Director of Singapore Volition and Hypergenomics on December 15, 2015. He has been a Director and Secretary of Belgian Volition since October 4, 2010 and was appointed Director of Volition Diagnostics UK Limited, on November 13, 2015. Mr. Rootsaert concurrently serves as director and corporate secretary of Mining House Ltd., positions he has had since 2007. His responsibilities include ensuring compliance with all relevant statutory and regulatory requirements. From 2007 until 2011, Mr. Rootsaert served as corporate secretary for Magellan Copper and Gold Plc., where his duties included maintaining and preparing company documents, accounts and contracts. With over ten years of experience in providing corporate, legal and administrative services and prior roles as corporate secretary for several mining companies in the United Kingdom, the Board of Directors believes that Mr. Rootsaert is a valuable addition to our team.
DR. JACOB MICALLEF serves as the Company’s Chief Scientific Officer. Dr. Micallef also served as a Director of Belgian Volition between August 10, 2011 and March 31, 2016. Prior to the Share Exchange Agreement, he served as a Science Executive Officer of Belgian Volition since October 11, 2010, but was not otherwise involved with Singapore Volition. Dr. Micallef joined Cronos Therapeutics Limited, or Cronos, in 2004 and, in 2006, Cronos was listed in the U.K. on the AIM, becoming Valirx plc, or Valirx. Dr. Micallef continued to work as Technical Officer for ValiRX, where he in-licensed the Hypergenomics® and Nucleosomics® technologies and co-founded ValiBio SA, which is now Belgian Volition. From 2004 to 2007, he taught “science and enterprise” to science research workers from four universities at CASS Business School before joining Cronos. In 2001, Dr. Micallef co-founded Gene Expression Technologies, after getting his MBA in 1999, where he successfully led the development of the chemistry of the GeneICE technology and implemented the manufacture of GeneICE molecules. He also played a major role in business development and procured a GeneICE contract with Bayer AG. Over a 15-year period, starting in 1985, Dr. Micallef worked for the World Health Organization, or WHO. While working for the WHO, Dr. Micallef developed new diagnostic products in the areas of reproductive health and cancer. In 1990, he commenced development of a new diagnostic technology platform for WHO which was launched in 1992 and supported 13 tests. Dr. Micallef also initiated and implemented in-house manufacture (previously outsourced to Abbott Diagnostics Inc.) and world-wide distribution of these products for WHO. Also in 1990, he started a “not-for-profit” WHO company, Immunometrics Ltd., which marketed and distributed those diagnostic products worldwide. Dr. Micallef has 20 years of experience in research and development and in the management of early stage biotechnical companies, including the manufacture of biotechnology products and the establishment of manufacturing operations. The Board of Directors believes that Dr. Micallef’s prior work with Belgian Volition in the development of diagnostic products would continue to be an asset to us in his role as Chief Scientific Officer of the Company and is subsidiaries.
30
DR. JASON TERRELL serves as our Chief Medical Officer and Head of U.S. Operations. Effective January 1, 2016, Dr. Terrell was appointed to the position of Chief Medical Officer and Head of U.S. Operations on a full-time basis, having previously served in a part-time capacity as the Company’s Chief Medical Officer and Head of U.S. Operations since March 2013. On February 3, 2017, Dr. Terrell was appointed Director and Chief Executive Officer of Volition America, Inc. Since February 2017, Dr. Terrell has also concurrently served as both a director and Chief Medical Diagnostics Officer of Generex Biotechnology Corporation (OTCMKTS : GNBT), a publicly-held biopharmaceutical company focused on developing or acquiring technologies that help pharmaceutical companies discover and develop medicines, and additionally as the non-executive chairman of the board of directors of Kiromic BioPharma, Inc. (a private company). Between January 2013 and October 2015, Dr. Terrell served on the board of directors of CDEX Inc., a publicly-held company developing drug validation technology, and between January 2012 and October 2015, as Medical Director of CDEX Inc. In addition, over the last six years, Dr. Terrell has built and sold multiple private diagnostic laboratories and currently serves as a National Franchise Corporate Medical Director for Any Lab Test Now, giving him oversight of over 70 franchises in 14 states. Dr. Terrell is a Texas-based doctor educated at the University of Texas and its affiliate MD Anderson Cancer Center, with expertise in both clinical medicine and the laboratory diagnostics business. He has a strong grounding in diagnostics and product commercialization and has both executive and board directorship experience with publicly traded companies in the biotechnology and pharmaceutical industries. Our Board of Directors has concluded that Dr. Terrell brings value to the Company with his strong grounding in both medicine and more specifically in diagnostics.
CORPORATE GOVERNANCE
Family Relationship
We currently do not have any officers or directors of our Company who are related to each other.
Involvement in Certain Legal Proceedings
During the past ten years no director, executive officer, promoter or control person of Volition or its subsidiaries, has been involved in any legal proceedings required to be disclosed pursuant to Item 401(f) of Regulation S-K.
Section 16(a) Beneficial Ownership Reporting Compliance
Section 16(a) of the Exchange Act requires our directors and executive officers and persons who beneficially own more than ten percent of a registered class of our equity securities to file with the SEC initial reports of ownership and reports of change in ownership of our common stock and other equity securities. Officers, directors and greater than ten percent stockholders are required by SEC regulations to furnish us with copies of all Section 16(a) forms they file.
Based solely upon a review of Forms 3, 4, and 5 and amendments thereto furnished to us under Rule 16a-3(e) during the year ended December 31, 2017, and the representations made by the reporting persons to us, we believe that during the year ended December 31, 2017, our executive officers and directors and all persons who own more than ten percent of a registered class of our equity securities have complied with all Section 16(a) filing requirements.
Code of Ethics
We have adopted a Code of Ethics, or the Code, that applies to our directors, officers and employees, including our Chief Executive Officer and Chief Financial Officer. A copy of the Code is available on our Company website at http://ir.volitionrx.com/governance-documents. Amendments to the Code that apply to our principal executive officer, principal financial officer, principal accounting officer, controller or persons performing similar functions, if any, will be posted on our website at http://ir.volitionrx.com/governance-documents. We will disclose any waivers of provisions of our Code that apply to such persons by disclosing such information on a Current Report on Form 8-K.
31
Committees of the Board of Directors
Our Board of Directors has established an audit committee, a compensation committee, and a nominations and governance committee. The committees operate pursuant to written charters adopted by the Board of Directors, copies of which are available on our website http://ir.volitionrx.com/committee-charters. In addition, from time to time, the Board of Directors may establish special committees when necessary to address specific issues.
Audit Committee
Our audit committee consists of four members, Mr. Innes (Chair), and Drs. Skaff, Colman and Futcher, each of whom has been determined to be an independent director under applicable SEC rules and the applicable rules of the NYSE American. The audit committee shall at all times be composed exclusively of directors who are, in the opinion of our Board of Directors, free from any relationship which would interfere with the exercise of independent judgment as a committee member and who possess an understanding of financial statements and generally accepted accounting principles.
The audit committee is responsible for, among other things:
appointing, terminating, compensating and overseeing the work of any independent auditor engaged to prepare or issue an audit report or other audit, review or attest services;
reviewing all audit and non-audit services to be performed by the independent auditor, taking into consideration whether the independent auditor’s provision of non-audit services to us is compatible with maintaining the independent auditor’s independence;
reviewing and discussing the adequacy and effectiveness of our accounting and financial reporting processes and internal controls and the audits of our financial statements;
establishing and overseeing procedures for the receipt, retention and treatment of complaints received by us regarding accounting, internal accounting controls or auditing matters, including procedures for the confidential, anonymous submission by our employees regarding questionable accounting or auditing matters;
investigating any matter brought to its attention within the scope of its duties and engaging independent counsel and other advisors as the audit committee deems necessary;
determining compensation of the independent auditors and of advisors hired by the audit committee and ordinary administrative expenses;
reviewing and discussing with management and the independent auditor the annual and quarterly financial statements prior to their release;
monitoring and evaluating the independent auditor’s qualifications, performance and independence on an ongoing basis;
reviewing reports to management prepared by the internal audit function, as well as management’s response;
reviewing and assessing the adequacy of the formal written charter on an annual basis; and
reviewing and approving related party transactions for potential conflict of interest situations on an ongoing basis; and overseeing such other matters that are specifically delegated to the audit committee by our Board of Directors from time to time.
The Board of Directors has affirmatively determined that Mr. Innes is designated as an “audit committee financial expert.”
Compensation Committee
Our compensation committee consists of three members, Mr. Innes (Chair) and Drs. Skaff and Futcher, each of whom has been determined to be an independent director under the applicable rules of the NYSE American.
The compensation committee is responsible for, among other things:
developing, reviewing, and approving our overall compensation programs, and regularly reporting to the full Board of Directors regarding the adoption of such programs;
developing, reviewing and approving our cash and equity incentive plans, including approving individual grants or awards thereunder;
reviewing and approving individual and company performance goals and objectives that may be relevant to the compensation of executive officers and other key employees;
reviewing and discussing with management the tables and narrative discussion regarding executive officer and director compensation to be included in the annual proxy statement;
32
reviewing and assessing, on an annual basis, the adequacy of the formal written charter; and
overseeing such other matters that are specifically delegated to the compensation committee by our Board of Directors from time to time.
In fulfilling its responsibilities, the compensation committee has the authority to delegate any or all of its responsibilities to a subcommittee of the compensation committee.
Nominations and Governance Committee
Our nominations and governance committee consists of three members, Mr. Innes (Chair) and Drs. Skaff and Futcher, each of whom has been determined to be an independent director under the applicable rules of the NYSE American.
The nominations and governance committee is responsible for, among other things:
identifying and screening candidates for our Board of Directors, and recommending nominees for election as directors;
assessing, on an annual basis, the performance of the Board of Directors and any committee thereof;
reviewing the structure of the Board of Director’s committees and recommending to the board for its approval directors to serve as members of each committee, including each committee’s respective chair, if applicable;
reviewing and assessing, on an annual basis, the adequacy of its formal written charter; and
generally advising our board of directors on corporate governance and related matters.
Nominating Procedures
The nominations and governance committee does not have a formal policy regarding the consideration of any director nominee, but will consider candidates for the Board of Directors from any reasonable source, including stockholder recommendations. The committee will not evaluate candidates differently based on who has made the proposal. The committee has the authority under its charter to hire and pay a fee to consultants or search firms to assist in the process of identifying and evaluating candidates. No such consultants or search firms have been used to date and, accordingly, no fees have been paid to consultants or search firms in the past fiscal year. The nominations and governance committee, and our Board of Directors, believe that directors should possess the highest personal and professional ethics, integrity and values, and to be committed to representing the long-term interests of the Company’s stockholders. Each director must also be able to dedicate the time and resources sufficient to ensure the diligent performance of his or her duties. Further, our Board of Directors is intended to encompass a range of talents, experience, skills, backgrounds, and expertise sufficient to provide sound and prudent guidance with respect to the operations and interests of the Company and its stockholders. The Company values diversity and seeks to achieve a diversity of professional experiences and personal backgrounds on our board of directors, but no specific policy regarding board diversity has been adopted.
Stockholders who wish to suggest qualified candidates should write to the chair of the nominations and governance committee at 1 Scotts Road, #24-05 Shaw Centre, Singapore 228208, specifying the name of the candidates and stating in detail the qualifications of such persons for consideration by the committee. A written statement from the candidate consenting to be named as a candidate and, if nominated and elected, to serve as a director should accompany any such recommendation.
33
ITEM 11.EXECUTIVE COMPENSATION
Summary Compensation Table
The following table sets forth the principal positions of our named executive officers at VolitionRX and the compensation paid to such persons for the fiscal years ended December 31, 2017 and 2016.
|
Year |
|
|
|
|
|
|
|
Ended |
|
Option |
All Other |
|
|
December |
Salary |
Awards |
Compensation |
Total |
|
|
Position |
31, |
($)(2) |
($)(1) |
($)(2) |
($) |
|
Cameron Reynolds(3) |
2017 |
293,974 |
303,672 |
92,364 |
690,010 |
|
President, CEO and Director |
2016 |
25,000 |
257,717 |
285,168 |
567,885 |
|
Dr Martin Faulkes(4) |
2017 |
178,447 |
258,349 |
-0- |
436.796 |
|
Executive Chairman |
2016 |
151,831 |
137,924 |
-0- |
289,755 |
|
Director | |||||
|
Dr Jacob Micallef(5) |
2017 |
140,926 |
240,898 |
37,577 |
419,401 |
|
Chief Scientific Officer |
2016 |
-0- |
260,178 |
151,551 |
411,729 |
(1)All option and warrant award amounts have been calculated based upon the aggregate grant date fair value computed in accordance with FASB ASC Topic 718.
(2)These amounts were paid in GBP Sterling at an average exchange rate for 2017 of $1.29 to £1 GBP, and for 2016 of $1.36 to £1 GBP, respectively.
(3)Mr. Reynold’s salary for the years ended December 31, 2017 and 2016 was determined pursuant to the Reynolds UK Employment Agreement and the Reynolds Executive Employment Agreement for the respective periods (as described in the section entitled “Employment and Consulting Agreements” below). On March 30, 2017 and April 15, 2016, Mr. Reynolds was granted options to purchase 100,000 shares and 125,000 shares, respectively, of common stock of VolitionRX under the 2015 Stock Incentive Plan, or 2015 Plan, vesting in full on the twelve-month anniversary of the date of grant. The amounts under All Other Compensation consist of consultancy fees received by Mr. Reynolds pursuant to the Reynolds Consultancy Agreement (as described in the section entitled “Employment and Consulting Agreements” below).
(4)Dr. Faulkes’ salary for the years ended December 31, 2017 and 2016 was determined pursuant to the Faulkes Employment Agreement and the Faulkes Executive Chairman Agreement, as amended, for the respective periods (as described in the section entitled “Employment and Consulting Agreements” below). On March 30, 2017 and April 15, 2016, Dr. Faulkes was granted options to purchase 100,000 shares and 65,000 shares, respectively, of common stock of VolitionRX under the 2015 Plan, vesting in full on the twelve-month anniversary of the date of grant.
(5)Dr. Micallef did not receive cash compensation directly from the Company prior to March 7, 2017, after which his salary for the year ended December 31, 2017 was determined pursuant to the Micallef UK Employment Agreement (as described in the section entitled “Employment and Consulting Agreements” below). On March 30, 2017 and April 15, 2016, Dr. Micallef was granted options to purchase 70,000 shares and 125,000 shares, respectively, of common stock of VolitionRX under the 2015 Plan, vesting in full on the twelve-month anniversary of the date of grant. The amounts under All Other Compensation consist of fees received under the 2015 Micallef Agreement, as amended (as described in the section entitled “Employment and Consulting Agreements” below).
34
Employment and Consulting Agreements
Cameron Reynolds
On March 7, 2017, Mr. Reynolds entered into an Employment Agreement with Volition Diagnostics, or the Reynolds UK Employment Agreement, which took effect on April 1, 2017 and replaced the Reynolds Executive Employment Agreement, the Reynolds Consultancy Agreement (as described below) and the 2016 PB Commodities Consulting Agreement (as referenced below). Pursuant to the terms of the Reynolds UK Employment Agreement, Mr. Reynolds shall serve as Chief Executive Officer of Volition Diagnostics. Volition Diagnostics will also make available the services of Mr. Reynolds, as Chief Executive Officer, to VolitionRX and its other subsidiaries, pursuant to services agreements entered into by and between Volition Diagnostics and VolitionRX or its subsidiaries. The Reynolds UK Employment Agreement continues until terminated by either party providing not less than six months’ notice. In exchange for his services, Mr. Reynolds shall receive, among other things (i) £24,500 GBP per month (approximately $30,224) from Volition Diagnostics; and (ii) a lump sum severance payment if terminated by Volition Diagnostics without cause (as per the agreement) equal to the salary that he would have received between the date of termination and the completion of a six month notice period. Effective from April 1, 2017, Mr. Reynolds additionally receives a payment of £1,225 GBP per month toward the Volition Diagnostics UK Group Personal Pension Plan (as described in detail below under “Long-Term Incentive Plans”). The foregoing description of the Reynolds UK Employment Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.27.
On May 11, 2016, Singapore Volition entered into a consultancy agreement with PB Commodities Pte. Limited, or PB Commodities, for the services of Cameron Reynolds, or the 2016 PB Commodities Consultancy Agreement (as further described in Item 13. Certain Relationships and Related Transactions, and Director Independence).
On January 1, 2015, Mr. Reynolds entered into a consultancy agreement with PB Commodities, or the Reynolds Consultancy Agreement. Mr. Reynolds received compensation from PB Commodities under the Reynolds Consultancy Agreement in exchange for serving as a consultant for PB Commodities and performing consultancy services on its behalf (including for services provided to Singapore Volition). In exchange for these services Mr. Reynolds received $21,085 per month during the fiscal year ended 2016 and until replaced by the Reynolds UK Employment Agreement. The foregoing description of the Reynolds Consultancy Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.12.
On January 1, 2015, Mr. Reynolds also entered into an Executive Employment Agreement with VolitionRX, or the Reynolds Executive Employment Agreement, in exchange for serving as the Chief Executive Officer of VolitionRX. The term of the Reynolds Executive Employment Agreement was three (3) years, which automatically extended for successive periods of two (2) years. In exchange for his services, Mr. Reynolds received $2,803 per month during the fiscal year ended 2016 and until replaced by the Reynolds UK Employment Agreement. Mr. Reynolds was also entitled to the use of a residential apartment in Namur, Belgium, as leased by the Company. The foregoing description of the Reynolds Executive Employment Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.13.
Dr. Martin Faulkes
On March 7, 2017, Dr. Faulkes entered into an Employment Agreement with Volition Diagnostics, or the Faulkes Employment Agreement, which took effect on April 1, 2017 and replaced the Faulkes Executive Chairman Agreement, as amended (as described below). Volition Diagnostics will make available to VolitionRX the services of Dr. Faulkes as Executive Chairman of the Board of VolitionRX, pursuant to a services agreement entered into by and between Volition Diagnostics and VolitionRX and subject to any necessary approval by the Company’s stockholders as required by applicable law and VolitionRX’s governing documents. The Faulkes Employment Agreement continues until terminated by either party providing not less than three months’ notice. In exchange for his services, Dr. Faulkes shall receive, among other things (i) £12,000 GBP per month from Volition Diagnostics; and (ii) a lump sum severance payment if terminated by Volition Diagnostics without cause (as per the agreement) equal to the salary that he would have received between the date of termination and the completion of a three month notice period. The foregoing description of the Faulkes Employment Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.30.
35
On March 31, 2015, Dr. Faulkes entered into an Executive Chairman Agreement with VolitionRX, or the Faulkes Executive Chairman Agreement, pursuant to which Dr. Faulkes will continue to serve as a member of the Board and as Executive Chairman of the Board of VolitionRX subject to any necessary approval by the Company’s stockholders as required by applicable law and VolitionRX’s governing documents. In exchange for his services Dr. Faulkes received £8,333 GBP per month commencing March 1, 2015. The foregoing description of the Faulkes Executive Chairman Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.21. On May 11, 2016, the Compensation Committee approved an amendment to the Faulkes Executive Chairman Agreement, or Amended Faulkes Executive Chairman Agreement. The Amended Faulkes Executive Chairman Agreement provided that Dr. Faulkes shall receive £10,000 GBP per month in exchange for his services. The foregoing description of the Amended Faulkes Executive Chairman Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.22.
Dr. Jacob Micallef
On March 7, 2017, Dr. Jacob Micallef entered into an Employment Agreement with Volition Diagnostics, or the Micallef UK Employment Agreement, which took effect on April 1, 2017 and replaced the 2015 Micallef Agreement, as amended (as described below). Volition Diagnostics will make available the services of Dr. Micallef, as Chief Scientific Officer, to VolitionRX and its other subsidiaries, pursuant to services agreements entered into by and between Volition Diagnostics and VolitionRX. The Micallef UK Employment Agreement continues until terminated by either party providing not less than three months’ notice. In exchange for his services, Dr. Micallef shall receive, among other things (i) £12,000 GBP per month from Volition Diagnostics; and (ii) a lump sum severance payment if terminated by Volition Diagnostics without cause (as per the agreement) equal to the salary that he would have received between the date of termination and the completion of a three month notice period. The foregoing description of the Micallef UK Employment Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.28.
On January 1, 2015, VolitionRX and Borlaug Limited or Borlang entered into consultancy agreement, or the 2015 Micallef Agreement. Under the terms of the 2015 Micallef Agreement, Borlaug made available to VolitionRX the services of Dr. Micallef to (i) manage VolitionRX’s intellectual property portfolio and file new patents as required by VolitionRX; (ii) provide project management for VolitionRX’s diagnostic development programs; and (iii) identify and pursue business development opportunities for VolitionRX. The 2015 Micallef Agreement continued until terminated in accordance with its terms. The foregoing description of the 2015 Micallef Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.14. On May 11, 2016, VolitionRX entered into an amendment of the 2015 Micallef Agreement. Pursuant to the amendment, Borlaug received £10,000 GBP per month in exchange for the services of Dr. Micallef. The foregoing description of the amendment to the 2015 Micallef Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.15.
36
Outstanding Equity Awards
The following table sets forth the outstanding equity awards for the named executive officers of VolitionRX as of the fiscal year ended December 31, 2017.
OUTSTANDING EQUITY AWARDS AT FISCAL YEAR-END
|
|
|
Option Awards
|
||||
|
Grant Date |
Number of Securities Underlying Unexercised Options (#) exercisable |
Number of Securities Underlying Unexercised Options (#) unexercisable |
Equity Incentive Plan Awards: Number of Securities Underlying Unexercised Unearned Options (#) |
Option Exercise Price ($) |
Option Expiration Date |
|
|
Cameron Reynolds |
November 25, 2011(1) |
40,000 |
-0- |
-0- |
(2) |
(3) |
|
|
|
|
|
|
|
|
|
|
August 18, 2014(4) |
100,000 |
-0- |
-0- |
(5) |
(6) |
|
|
|
|
|
|
|
|
|
|
July 23, 2015(7) |
55,000 |
-0- |
-0- |
$4.00 |
January 23, 2020 |
|
|
|
|
|
|
|
|
|
|
April 15, 2016(8) |
125,000 |
-0- |
-0- |
$4.00 |
April 15, 2022 |
|
|
|
|
|
|
|
|
|
|
March 30, 2017 (9) |
-0- |
100,000 |
-0- |
$5.00 |
March 30, 2023 |
|
Dr. Jacob Micallef |
November 25, 2011(10) |
40,000 |
-0- |
-0- |
(2) |
(3) |
|
|
|
|
|
|
|
|
|
|
August 18, 2014(11) |
130,000 |
-0- |
-0- |
(5) |
(12) |
|
|
|
|
|
|
|
|
|
|
July 23, 2015(13) |
55,000 |
-0- |
-0- |
$4.00 |
January 23, 2020 |
|
|
|
|
|
|
|
|
|
|
April 15, 2016(14) |
125,000 |
-0- |
-0- |
$4.00 |
April 15, 2022 |
|
|
|
|
|
|
|
|
|
|
March 30, 2017 (15) |
-0- |
70,000 |
-0- |
$5.00 |
March 30, 2023 |
|
Martin Faulkes |
November 25, 2011(16) |
10,000 |
-0- |
-0- |
(2) |
(17) |
|
|
|
|
|
|
|
|
|
|
August 18, 2014(18) |
60,000 |
-0- |
-0- |
(5) |
(19) |
|
|
|
|
|
|
|
|
|
|
July 23, 2015(20) |
40,000 |
-0- |
-0- |
$4.00 |
January 23, 2020 |
|
|
|
|
|
|
|
|
|
|
April 15, 2016(21) |
65,000 |
-0- |
-0- |
$4.00 |
April 15, 2022 |
|
|
|
|
|
|
|
|
|
|
March 30, 2017 (22) |
-0- |
100,000 |
-0- |
$5.00 |
March 30, 2023 |
|
|
|
|
|
|
|
|
37
(1)On November 25, 2011, Mr. Reynolds was granted an option to purchase 120,000 shares of common stock of VolitionRX under the 2011 Plan vesting one-sixth (20,000 shares) every 6 months from the date of grant. As of the date of this report, options to purchase 40,000 shares have been exercised and options to purchase 40,000 shares expired unexercised.
(2)Pursuant to the applicable option agreement, as amended, the exercise price of the options is $5.00 per share for those vesting on May 25, 2014 and November 25, 2014.
(3)Pursuant to the applicable option agreement, as amended, the expiration date of the options is four years from the date of vesting, such that options to purchase 20,000 shares expire on each of May 25, 2018 and November 25, 2018.
(4)On August 18, 2014, Mr. Reynolds was granted an option to purchase 100,000 shares of common stock of VolitionRX under the 2011 Plan, vesting one-half (50,000 shares) on the six month anniversary of the date of grant and vesting one-half (50,000 shares) on the eighteen month anniversary of the date of grant.
(5)Pursuant to the applicable option agreement, the exercise price of the options is (a) $2.50 per share for those vesting on February 18, 2015 and (b) $3.00 per share for those vesting on February 18, 2016.
(6)Pursuant to the applicable option agreement, the expiration date of the options is four years from the date of vesting, such that options to purchase 50,000 shares expire on each of February 18, 2019 and February 18, 2020.
(7)On July 23, 2015, Mr. Reynolds was granted an option to purchase 55,000 shares of common stock of VolitionRX under the 2011 Plan, vesting in full on the six month anniversary of the date of grant.
(8)On April 15, 2016, Mr. Reynolds was granted an option to purchase 125,000 shares of common stock of VolitionRX under the 2015 Plan vesting in full on the twelve month anniversary of the date of grant.
(9)On March 30, 2017, Mr. Reynolds was granted an option to purchase 100,000 shares of common stock of VolitionRX under the 2015 Plan, vesting in full on the twelve month anniversary of the date of grant.
(10)On November 25, 2011, Dr. Micallef was granted an option to purchase 120,000 shares of common stock of VolitionRX under the 2011 Plan, vesting one-sixth (20,000 shares) every 6 months from the date of grant. This option has been subsequently transferred to Borlaug for no value. As of the date of this report, options to purchase 40,000 shares have been exercised and options to purchase 40,000 shares have expired unexercised.
(11)On August 18, 2014, Borlaug was granted an option to purchase 130,000 shares of common stock of VolitionRX under the 2011 Plan, vesting one-half (65,000 shares) on the six month anniversary of the date of grant and vesting one-half (65,000 shares) on the eighteen month anniversary of the date of grant. This option has been granted to Borlaug for the services of Dr. Micallef.
(12)Pursuant to the applicable option agreement, the expiration date of the options is four years from the date of vesting, such that options to purchase 65,000 shares expire on each of February 18, 2019 and February 18, 2020.
(13)On July 23, 2015, Borlaug was granted an option to purchase 55,000 shares of common stock of VolitionRX under the 2011 Plan, vesting in full on the six month anniversary of the date of grant. This option has been granted to Borlaug for the services of Dr. Micallef.
(14)On April 15, 2016, Dr. Micallef was granted an option to purchase 125,000 shares of common stock of VolitionRX under the 2015 Plan, vesting in full on the twelve month anniversary of the date of grant. This option has been subsequently transferred to Borlaug for no value.
(15)On March 30, 2017, Dr. Micallef was granted an option to purchase 70,000 shares of common stock of VolitionRX under the 2015 Plan, vesting in full on the twelve month anniversary of the date of grant.
(16)On November 25, 2011, Dr. Faulkes was granted an option to purchase 30,000 shares of common stock of VolitionRX under the 2011 Plan, vesting one-sixth (5,000 shares) every 6 months from the date of grant. As of the date of this report, options to purchase 10,000 shares have been exercised and options to purchase 10,000 shares expired unexercised.
(17)Pursuant to the applicable option agreement, the expiration date of the options is four years from the date of vesting, such that options to purchase 5,000 shares expire on each of May 25, 2018 and November 25, 2018.
38
(18)On August 18, 2014, Dr. Faulkes was granted an option to purchase 60,000 shares of common stock of VolitionRX under the 2011 Plan, vesting one-half (30,000 shares) on the six month anniversary of the date of grant and vesting one-half (30,000 shares) on the eighteen month anniversary of the date of grant.
(19)Pursuant to the applicable option agreement, the expiration date of the options is four years from the date of vesting, such that options to purchase 30,000 shares expire on each of February 18, 2019 and February 18, 2020.
(20)On July 23, 2015, Dr. Faulkes was granted an option to purchase 40,000 shares of common stock of VolitionRXx under the 2011 Plan, vesting in full on the six month anniversary of the date of grant.
(21)On April 15, 2016, Dr. Faulkes was granted an option to purchase 65,000 shares of common stock of VolitionRX under the 2015 Plan, vesting in full on the twelve month anniversary of the date of grant.
(22)On March 30, 2017, Dr. Faulkes was granted an option to purchase 100,000 shares of common stock of VolitionRX under the 2015 Plan, vesting in full on the twelve month anniversary of the date of grant.
Long-Term Incentive Plans
Volition Diagnostics UK operates a Group Personal Pension Plan, or the “Pension Plan”, and makes defined monthly contributions into a separate fund on behalf of its eligible United Kingdom employees, as required by the Pensions Act 2008 (UK). Certain of the Company’s executive officers who are based in the United Kingdom are eligible to participate in the Pension Plan. Volition Diagnostics UK contributes five percent of the gross salary paid to those of its eligible employees to the Pension Plan. Those eligible employees are also required to contribute to the Pension Plan. All risks associated with this type of plan are assumed by the employees. The Pension Plan was effective commencing April 6, 2017.
Other than the foregoing, there are no arrangements or plans in which VolitionRX, Singapore Volition or its subsidiaries provided pension, retirement or similar benefits for directors or executive officers.
Compensation of Directors
The following table sets forth the compensation paid to the non-employee directors of VolitionRX for the fiscal year ended December 31, 2017. No executive officer is paid compensation for his role as a director. There are no employment agreements by and between the Company and the non-employee directors. See the sections entitled “Executive Compensation – Summary Compensation Table” for additional information on the compensation paid to executive offers who were also directors.
Director Compensation Table
|
Name |
Fees Earned or Paid in Cash ($) |
Stock Awards ($) |
Option Awards(1) ($) |
Non-Equity Incentive Plan Compensation ($) |
Nonqualified Deferred Compensation Earnings ($) |
All Other Compensation ($) |
Total ($) |
|
Guy Innes(2) |
40,000 |
-0- |
64,511 |
-0- |
-0- |
-0- |
104,511 |
|
Dr. Alan Colman(3) |
60,000 |
-0- |
56,957 |
-0- |
-0- |
-0- |
116,957 |
|
Dr. Habib Skaff(4) |
40,000 |
-0- |
42,718 |
-0- |
-0- |
-0- |
82,718 |
|
Dr. Edward Futcher(5) |
40,000 |
-0- |
47,566 |
-0- |
-0- |
-0- |
87,566 |
(1)All option awards have been calculated based upon the aggregate grant date fair value computed in accordance with FASB ASC Topic 718. The Company has calculated the estimated fair market value of these options granted on March 30, 2017, using the Black-Scholes model and the following assumptions: term 6 years; stock price: $4.18; exercise price: $5.00; 79.41% volatility; 2.25% risk free rate.
(2)On March 31, 2015 Mr. Innes entered into an Independent Director Agreement with VolitionRX, or the Innes Independent Director Agreement, pursuant to which Mr. Innes will continue to serve as a member of the Board of VolitionRX subject to any necessary approval by the Company’s stockholders as required by applicable law and VolitionRX’s governing documents. In exchange for his services Mr Innes shall receive $10,000 per calendar quarter commencing March 1, 2015. The foregoing description of the Innes Independent Director Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.23.
39
On March 30, 2017, Mr. Innes was granted an option to purchase 20,000 shares of common stock of VolitionRX under the 2015 Plan, vesting in full on the twelve-month anniversary of the date of grant.
(3)On March 31, 2015, Dr. Colman entered into an Independent Director Agreement with VolitionRX, or the Colman Independent Director Agreement, pursuant to which Dr. Colman will continue to serve as a member of the Board of VolitionRX subject to any necessary approval by the Company’s stockholders as required by applicable law and VolitionRX’s governing documents. In exchange for his services Dr. Colman shall receive $15,000 per calendar quarter commencing March 1, 2015. The foregoing description of the Colman Independent Director Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.23.
On March 30, 2017, Dr. Colman was granted an option to purchase 20,000 shares of common stock of VolitionRX under the 2015 Plan, vesting in full on the twelve-month anniversary of the date of grant.
(4)On March 31, 2015, Dr. Skaff entered into an Independent Director Agreement with VolitionRX, or the Skaff Independent Director Agreement, pursuant to which Dr. Skaff will continue to serve as a member of the Board of VolitionRX subject to any necessary approval by the Company’s stockholders as required by applicable law and VolitionRX’s governing documents. In exchange for his services Dr. Skaff shall receive $10,000 per calendar quarter commencing March 1, 2015. The foregoing description of the Skaff Independent Director Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.23.
On March 30, 2017, Dr. Skaff was granted an option to purchase 15,000 shares of common stock of VolitionRX under the 2015 Plan, vesting in full on the twelve month anniversary of the date of grant.
(5)On June 23, 2016 Dr. Futcher entered into an Independent Director Agreement with VolitionRX, or the Futcher Independent Director Agreement, pursuant to which Dr. Futcher will continue to serve as a member of the Board of VolitionRX subject to any necessary approval by the Company’s stock holders as required by applicable law and VolitionRX’s governing documents. In exchange for his services, Dr. Futcher shall receive $10,000 per calendar quarter commencing June 23, 2016. The foregoing description of the Futcher Independent Director Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.23.
On March 30, 2017, Dr. Futcher was granted an option to purchase 15,000 shares of common stock of VolitionRX under the 2015 Plan, vesting in full on the twelve-month anniversary of the date of grant.
Stockholder Recommendations to the Board of Directors
Stockholders can direct communications to our Secretary, Rodney Rootsaert, at our executive offices, located at 1 Scotts Road, #24-05 Shaw Centre, Singapore 228208. However, while we appreciate all comments from stockholders, we may not be able to individually respond to all communications. We attempt to address stockholder questions and concerns in our press releases and documents filed with the SEC so that all stockholders have access to information about us at the same time. Mr. Rootsaert collects and evaluates all stockholder communications. All communications addressed to our directors and executive officers will be reviewed by those parties unless the communication is clearly frivolous.
40
ITEM 12.SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS
Security Ownership of Management
The following table sets forth certain information concerning the number of shares of our common stock owned beneficially as of February 27, 2018, by: (i) each of our directors and director nominees; (ii) each of our named executive officers; (iii) all of our directors, director nominees and executive officers as a group; and (iv) each person or group known by us to beneficially own more than 5% of our outstanding shares of common stock.
We have determined beneficial ownership in accordance with the rules of the SEC and the information is not necessarily indicative of beneficial ownership for any other purpose. Under the rules of the SEC, a person is deemed to be a beneficial owner of a security if that person has or shares “voting power,” which includes the power to vote or to direct the voting of such security, or investment power, which includes the power to dispose of or to direct the disposition of such security. A person is also deemed to be a beneficial owner of any securities of which the person has a right to acquire beneficial ownership within sixty (60) days. Under these rules more than one person may be deemed a beneficial owner of the same securities and a person may be deemed to be a beneficial owner of securities as to which such person has no economic interest.
Unless otherwise indicated below, to the best of our knowledge (i) each beneficial owner named in the table has the sole voting and sole investment power with respect to all shares beneficially owned, subject to community property laws where applicable, and (ii) the address of such beneficial owner is 1 Scotts Road, #24-05 Shaw Centre, Singapore, 228208.
|
Name and Address of Beneficial Owner |
Amount and Nature Of Beneficial Ownership (#) |
Percent of Class (1) (%) |
|
Directors and Named Executive Officers: |
|
|
|
Dr. Alan Colman(2) |
216,250 |
* |
|
Dr. Martin Faulkes(3) |
1,982,284 |
7.4% |
|
Dr. Edward Futcher(4) |
413,000 |
1.6% |
|
Guy Innes(5) |
1,591,947 |
6.0% |
|
Dr. Jacob Micallef(6) |
580,569 |
2.2% |
|
Cameron Reynolds(7) |
2,576,467 |
9.6% |
|
Rodney Rootsaert(8) |
1,252,546 |
4.7% |
|
Habib Skaff(9) |
92,542 |
* |
|
Jason Terrell(10) |
186,364 |
* |
|
David Vanston(11) |
102,750 |
* |
|
Other Executive Officers(12) |
1,178,478 |
4.3% |
|
All Executive Officers and Directors as a Group (17 Persons)(13) |
8,809,479 |
29.9% |
|
5% Stockholders: |
|
|
|
Cotterford Company Limited(14) Hever Investments Limited Alma House, 7 Circular Road, Douglas Isle of Man, IM1 1AF United Kingdom |
1,447,616 |
5.4% |
|
Richard Bayles (15) Fatina Dickey Youngdawn Ha Lagoda Investment Management, L.P. 3 Columbus Circle New York, New York |
3,516,963 |
13.3% |
(1)For purposes of the table, the percent of class is based upon 26,530,793 shares of our common stock issued and outstanding as of February 27, 2018. Shares of common stock subject to stock purchase options or warrants currently exercisable, or exercisable within 60 days of February 27, 2018, are deemed beneficially owned and outstanding for computing the percentage of the person or entity holding such securities, but are not considered outstanding for computing the percentage of any other person or entity.
(2)Dr. Colman’s beneficial ownership includes direct ownership of (i) 156,250 shares of common stock and (ii) options to purchase 60,000 shares of common stock that are exercisable within 60 days.
41
(3)Dr. Faulkes’s beneficial ownership includes direct ownership of (i) 1,351,284 shares of common stock and (ii) options to purchase 275,000 shares of common stock that are exercisable within 60 days. Dr. Faulkes’s beneficial ownership also includes indirect ownership of 356,000 shares of common stock held directly by The Dill Faulkes Educational Trust Limited, or DFET. Dr. Faulkes serves as the chairman, director and trustee of the DFET and shares voting and dispositive control over such shares. On December 8, 2015, Dr. Faulkes pledged 12,500 shares to secure a loan.
(4)Dr. Futcher’s beneficial ownership includes direct ownership of (i) 27,000 shares of common stock and (ii) options to purchase 30,000 shares of common stock that are exercisable within 60 days. Dr. Futcher’s beneficial ownership also includes indirect ownership of 356,000 shares of common stock held directly by DFET. Dr. Futcher serves as a director and a trustee of DFET and shares voting and dispositive control over such shares.
(5)Mr. Innes’s beneficial ownership includes direct ownership of (i) 1,304,975 shares of common stock; (ii) options to purchase 105,000 shares of common stock that are exercisable within 60 days; and (iii) warrants to purchase 132,246 shares of Company common stock that are exercisable within 60 days. Mr. Innes’s beneficial ownership also includes indirect ownership of 49,726 shares of Company common stock which are held in a bare trust, which is not a separate legal entity, of which Mr. Innes is the trustee, for the benefit of certain minors.
(6)Dr. Micallef’s beneficial ownership includes direct ownership of (i) 86,166 shares of common stock, (ii) options to purchase 70,000 shares of common stock that are exercisable within 60 days, and (iii) warrants to purchase 10,000 shares of common stock that are exercisable within 60 days. Dr. Micallef’s beneficial ownership also includes indirect ownership of (v) 11,000 shares of common stock held directly by Dr. Micallef’s wife, (w) 38,113 shares of common stock held directly by Borlaug, which Dr. Micallef shares voting and dispositive control over, (x) warrants held directly by Dr. Micallef’s wife to purchase 11,000 shares of common stock that are exercisable within 60 days, (y) warrants held directly by Borlaug to purchase 4,290 shares of common stock that are exercisable within 60 days, and (z) options held directly by Borlaug to purchase 350,000 shares of common stock that are exercisable within 60 days.
(7)Mr. Reynolds’s beneficial ownership includes direct ownership of (i) 1,114,673 shares of common stock and (ii) options to purchase 420,000 shares of common stock that are exercisable within 60 days. Mr. Reynolds’s beneficial ownership also includes indirect ownership of (x) 34,076 shares of common stock held directly by Mr. Reynolds’s spouse and (y) 1,007,718 shares of common stock held directly by Concord International, Inc., of which Mr. Reynolds’s is the majority shareholder and shares voting and dispositive control over such shares.
(8)Mr. Rootsaert’s beneficial ownership includes direct ownership of (i) 4,828 shares of common stock and (ii) options to purchase 240,000 shares of common stock that are exercisable within 60 days. Mr. Rootsaert’s beneficial ownership also includes indirect ownership of 1,007,718 shares of common stock beneficially owned by Concord International, Inc., for which Mr. Rootsaert serves as a controlling director and shares voting and dispositive control over such shares.
(9)Dr. Skaff’s beneficial ownership includes direct ownership of (i) 19,542 shares of common stock and (ii) options to purchase 73,000 shares of common stock that are exercisable within 60 days.
(10)Dr. Terrell’s beneficial ownership includes direct ownership of (i) 61,364 shares of common stock, (ii) options to purchase 100,000 shares of common stock that are exercisable within 60 days and (iii) warrants to purchase 25,000 shares of common stock that are exercisable within 60 days.
(11)Mr. Vanston’s beneficial ownership includes direct ownership of (i) 2,750 shares of common stock and (ii) options to purchase 100,000 shares of common stock that are exercisable within 60 days.
(12)The other executive officers of the Company have beneficial ownership of 259,435 shares of common stock, 898,634 shares issuable upon the exercise of stock purchase options, and 20,409 shares issuable upon the exercise of stock purchase warrants.
(13)The number of executive officers, directors and director nominees as a group includes seven executive officers of the Company’s subsidiaries. The amount beneficially owned by the executive officers, directors and director nominees as a group consists of an aggregate of 5,884,900 shares of common stock, 2,721,634 shares issuable upon the exercise of stock purchase options, and 202,945shares issuable upon the exercise of stock purchase warrants.
(14)Cotterford Company Limited and Hever Investments Limited together beneficially own 1,143,463 shares of common stock, and 304,153 shares issuable upon the exercise of stock purchase warrants. Jack Murphy holds dispositive and voting control over the shares of common stock beneficially owned by both Cotterford Company Limited and Hever Investments Limited.
(15)This information has been derived from a Schedule 13G/A filed with the SEC on February 14, 2018. Based on the information contained in the filing, Lagoda Investment Management, L.P. serves as the investment manager to certain managed accounts, and Richard Bayles, Fatima Dickey and Youngdawn Daniel Ha, as the managing principals of Lagoda Investment Management, LLC, the General Partner of Lagoda Investment Management, L.P. possess sole voting and dispositive power with respect to the common stock.
Changes in Control
There are no present arrangements or pledges of the Company’s securities which may result in a change in control of the Company.
42
Securities Authorized for Issuance Under Equity Compensation Plans
Under the 2015 Plan, we may grant incentive awards, including options, restricted stock, stock bonuses, stock appreciation rights, restricted stock units or performance awards, to any qualified employee, officer, director, consultant or other service provider that provides services to us or any of our affiliates. An aggregate of 2,500,000 shares of our common stock are reserved for issuance under the 2015 Plan. The purpose of the 2015 Plan is to provide additional incentives to eligible participants to devote their utmost effort and skill to the advancement and betterment of the registrant, by providing them an opportunity to participate in the ownership of the registrant and thereby have an interest in the success and increased value of the Company. The 2015 Plan replaces the 2011 Plan which was also approved by the stockholders. No further grants will be made under the 2011 Plan.
|
Plan category |
|
Number of Securities to be issued upon exercise of outstanding options, warrants and rights (a) |
|
Weighted-average exercise price of outstanding options, warrants and rights (b) |
|
Number of securities remaining available for future issuance under equity compensation plans (excluding securities reflected in column (a)) |
|
Equity compensation plans approved by security holders: - 2011 Equity Incentive Plan |
|
1,268,134 |
|
$3.51 |
|
-0- |
|
- 2015 Stock Incentive Plan |
|
1,671,000 |
|
$4.52 |
|
829,000 |
|
Equity compensation plans not approved by security holders |
|
-0- |
|
-0- |
|
-0- |
|
Total |
|
2,939,134 |
|
$4.09 |
|
829,000 |
ITEM 13.CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE
See Item 11. Executive Compensation – Employment and Consulting Agreements of this report.
On May 11, 2016, Singapore Volition entered into a consultancy agreement with PB Commodities Pte. Limited, or PB Commodities, for the services of Cameron Reynolds, or the 2016 PB Commodities Consultancy Agreement. Under the terms of the 2016 PB Commodities Consulting Agreement, PB Commodities received net $25,417 per month for the services provided to Singapore Volition by Mr. Reynolds on its behalf. For the years ended December 31, 2017 and 2016, PB Commodities received $76,251 and $285,168, respectively, pursuant to the 2016 PB Commodities Consultancy Agreement. The 2016 PB Commodities Consulting Agreement was terminated on March 31, 2017. The foregoing description of the 2016 PB Commodities Consultancy Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.5 filed herewith.
On May 2, 2014, VolitionRX entered into a consultancy agreement with Isosceles Finance Limited, or Isosceles, a consulting services company founded by VolitionRX’s then-current Chief Financial Officer Mike O’Connell, for the provision of accountancy and financial control services, or the Isosceles Consultancy Agreement. The initial term of the Isosceles Consultancy Agreement was for twelve (12) months, with automatic extensions for successive twelve (12) month periods until terminated as provided in the Agreement. While Mr. O’Connell ceased serving as VolitionRX’s Chief Financial Officer in August 2015, the Isosceles Consultancy Agreement continues in place. The services are provided on a time and materials basis. For the years ended December 31, 2017 and 2016, Isosceles received $262,310 and $273,507 respectively, pursuant to the Isosceles Consultancy Agreement. The foregoing description of the Isosceles Consultancy Agreement does not purport to summarize all terms and conditions thereof and is qualified in its entirety by reference to Exhibit 10.26.
As part of the engagement letters with each of our directors, certain indemnification provisions may require us, among other things, to indemnify our directors and executive officers for expenses, including attorneys’ fees, judgments, fines and settlement amounts incurred by a director or officer in any action or proceeding arising out of his or her service as one of our directors or officers.
Other than the foregoing, none of the directors or executive officers of the Company, nor any person who owned of record or was known to own beneficially more than 5% of the Company’s outstanding shares of its Common Stock, nor any associate or affiliate of such persons or companies, has any material interest, direct or indirect, in any transaction that has occurred during the past two fiscal years, or in any proposed transaction, which has materially affected or will affect the Company.
43
Director Independence
For purposes of determining director independence, the Board of Directors reviews a summary of the relationships of each director with the Company and other facts relevant to the analysis of whether the directors qualify as “independent directors” under the NYSE American Company Guide §803(A)(2). No director qualifies as independent unless the Board of Directors affirmatively determines that the director does not have a relationship that would interfere with the exercise of independent judgment in carrying out the responsibilities of a director. In addition, the NYSE American Company Guide provides a non-exclusive list of persons who may not be considered independent.
The Board of Directors has affirmatively determined that each of Drs. Colman, Futcher and Skaff, as well as Mr. Innes, is an independent director under the rules of the NYSE American. In addition, the members of the Audit Committee are independent directors pursuant to the heightened independence criteria for members of Audit Committees set forth in SEC rules.
Policy on the Review, Approval or Ratification of Transactions with Related Persons
The Company has not adopted a separate written policy for the approval or ratification of all transactions with related parties that are required to be reported under Item 404(a) of Regulation S-K. Rather, at this time and pursuant to its existing charter, and unless otherwise provided by the Board of Directors, the Audit Committee of the Board of Directors reviews the material facts of all such transactions and either ratifies, approves or disapproves of the entry into the transaction.
No director is allowed to participate in the approval of a transaction for which he or she is a related party and the director has to provide all material information concerning the transaction to the Audit Committee.
ITEM 14.PRINCIPAL ACCOUNTANT FEES AND SERVICES
|
|
|
Year Ended December 31, 2017 |
|
|
Year Ended December 31, 2016 |
|
Audit fees |
$ |
41,428 |
|
$ |
39,000 |
|
Audit-Related fees |
$ |
-0- |
|
$ |
1,240 |
|
Tax fees |
$ |
1,593 |
|
$ |
4,250 |
|
All other fees |
$ |
-0- |
|
$ |
-0- |
|
Total |
$ |
43,021 |
|
$ |
44,490 |
Audit Fees
Represents the aggregate fees billed to us for each of the last two fiscal years for professional services rendered by the principal accountant for the audit of our annual financial statements and review of financial statements included in our Form 10-Q or services that are normally provided by the accountant in connection with statutory and regulatory filings or engagement for those fiscal years.
Audit-Related Fees
Represents the aggregate fees billed to us in each of the last two fiscal years for assurance and related services by the principal accountants that are reasonably related to the performance of the audit or review of our financial statements that are not already reported in Audit Fees. These services include accounting consultations and attestation services that are not required by statute.
Tax Fees
Represents the aggregate fees billed to us in each of the last two fiscal years for professional services rendered by the principal account for tax compliance, tax advice, and tax planning.
All Other Fees
Represents the aggregate fees billed in each of the last two fiscal years for products and services provided by the principal accountant to us, excluding those enumerated above.
Policy on Audit Committee Pre-approval of Audit and Permissible Non-audit Services of Independent Auditor
All audit and non-audit services by our independent registered public accounting firm are pre-approved by our audit committee. For audit services, the independent accountant provides the Audit Committee with an audit plan, including proposed fees in advance of the annual audit. The audit committee approves the plan and fees for the audit.
Pursuant to its charter, the audit committee may establish pre-approval policies and procedures, subject to SEC and NYSE American rules and regulations, to approve audit and non-audit services; however, it has not yet done so.
44
PART IV
ITEM 15.EXHIBITS, FINANCIAL STATEMENT SCHEDULES
(a)The following documents are filed as part of this report:
1.Financial Statements. Included in Part II, Item 8 of this report and are incorporated by reference herein.
2.Financial Statement Schedules. Financial statement schedules are omitted because they are not applicable or the required information is shown in the financial statements or notes thereto.
3.Exhibits.
|
|
|
|
|
Incorporated by Reference |
|
|||||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed Herewith |
||
|
|
Share Purchase Agreement by and between Singapore Volition and ValiRX dated September 22, 2010.
|
|
8-K/A |
|
000-30402 |
|
2.01 |
|
5/8/12 |
|
|
|||
|
|
Supplementary Agreement to the Share Purchase Agreement by and between Singapore Volition and ValiRX dated June 9, 2011.
|
|
8-K/A |
|
000-30402 |
|
10.15 |
|
1/11/12 |
|
|
|||
|
|
Share Exchange Agreement by and among Standard Capital Corporation, the controlling shareholders of Standard Capital Corporation and Singapore Volition dated September 26, 2011.
|
|
8-K |
|
000-30402 |
|
2.1 |
|
9/29/11 |
|
|
|||
|
|
Agreement, Consent and Waiver by and between Standard Capital Corporation and its Shareholders dated September 27, 2011.
|
|
8-K/A |
|
000-30402 |
|
10.28 |
|
4/5/12 |
|
|
|||
|
|
Second Amended and Restated Certificate of Incorporation, as currently in effect.
|
|
8-K |
|
001-36833 |
|
3.1 |
|
10/11/16 |
|
|
|||
|
|
Amended and Restated Bylaws, as currently in effect.
|
|
S-8 |
|
333-208512 |
|
4.2 |
|
12/11/15 |
|
|
|||
|
|
Patent License Agreement by and between ValiRX and Chroma dated October 3, 2007.
|
|
8-K/A |
|
000-30402 |
|
10.04 |
|
1/11/12 |
|
|
|||
|
|
Contract Repayable Grant Advance on the Diagnosis of Colorectal Cancer by “NucleosomicsTM” by and between ValiBio SA and The Walloon Region dated December 17, 2009.
|
|
8-K/A |
|
000-30402 |
|
10.05 |
|
2/24/12 |
|
|
|||
|
|
Non-Exploitation and Third Party Patent License Agreement by and among ValiBio SA, ValiRX and The Walloon Region dated December 17, 2009.
|
|
8-K/A |
|
000-30402 |
|
10.06 |
|
2/24/12 |
|
|
|||
|
10.4# |
|
Agreement by and between Singapore Volition and PB Commodities dated August 6, 2010.
|
|
8-K/A |
|
000-30402 |
|
10.07 |
|
1/11/12 |
|
|
||
|
10.5# |
|
Consultancy Agreement by and between Singapore Volition and PB Commodities, dated May 11, 2016. |
|
10-Q |
|
001-36833 |
|
10.2 |
|
5/13/16 |
|
|
||
45
|
|
|
|
|
Incorporated by Reference |
|
|||||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed Herewith |
||
|
|
Deed of Novation by and among Singapore Volition, Valirx, ValiBio SA and Chroma dated September 22, 2010.
|
|
8-K/A |
|
000-30402 |
|
10.09 |
|
2/24/12 |
|
|
|||
|
|
Patent License Agreement by and between Singapore Volition and Belgian Volition dated November 2, 2010.
|
|
8-K/A |
|
000-30402 |
|
10.12 |
|
1/11/12 |
|
|
|||
|
|
License Agreement by and between Singapore Volition and the European Molecular Biology Laboratory dated June 6, 2011.
|
|
8-K/A |
|
000-30402 |
|
10.14 |
|
1/11/12 |
|
|
|||
|
10.9# |
|
Agreement by and between Hypergenomics and PB Commodities dated October 1, 2011.
|
|
8-K/A |
|
000-30402 |
|
10.27 |
|
2/24/12 |
|
|
||
|
|
Agreement by and between Belgian Volition and the Biobank of CHU UCL Mont-Godinne dated August 6, 2012.
|
|
S-1/A |
|
333-183056 |
|
10.27 |
|
10/4/12 |
|
|
|||
|
|
Common Stock Purchase Agreement, by and among VolitionRX and the purchasers thereto dated February 26, 2014.
|
|
8-K |
|
000-30402 |
|
10.1 |
|
2/28/14 |
|
|
|||
|
|
Consultancy Agreement by and between PB Commodities and Cameron Reynolds effective as of January 1, 2015.
|
|
S-1/A |
|
333-200628 |
|
10.25 |
|
1/8/15 |
|
|
|||
|
|
Executive Employment Agreement by and between VolitionRX and Cameron Reynolds effective as of January 1, 2015.
|
|
S-1/A |
|
333-200628 |
|
10.26 |
|
1/23/15 |
|
|
|||
|
|
Consultancy Agreement by and between VolitionRX and Borlaug dated as of January 1, 2015.
|
|
S-1/A |
|
333-200628 |
|
10.27 |
|
1/23/15 |
|
|
|||
|
|
First Amendment to Consultancy Agreement by and between VolitionRX and Borlaug, dated May 11, 2016.
|
|
10-Q |
|
001-36833 |
|
10.3 |
|
5/13/16 |
|
|
|||
|
|
Employment Agreement by and between VolitionRX and Rodney Rootsaert effective as of January 1, 2015.
|
|
S-1/A |
|
333-200628 |
|
10.28 |
|
1/23/15 |
|
|
|||
|
|
Employment Agreement by and between VolitionRX and Jason Terrell MD, dated December 29, 2015.
|
|
10-K |
|
001-36833 |
|
10.24 |
|
3/11/16 |
|
|
|||
|
|
Employment Agreement by and between VolitionRX and David Kratochvil dated August 11, 2015.
|
|
10-K |
|
001-36833 |
|
10.25 |
|
3/11/16 |
|
|
|||
|
|
2011 Equity Incentive Plan dated November 17, 2011.
|
|
8-K |
|
000-30402 |
|
4.01 |
|
11/18/11 |
|
|
|||
|
10.19(a)# |
|
Form Stock Option Agreement.
|
|
8-K |
|
000-30402 |
|
4.02 |
|
11/18/11 |
|
|
||
|
10.19(b)# |
|
Form Stock Award Agreement for Restricted Stock. |
|
8-K |
|
000-30402 |
|
4.03 |
|
11/18/11 |
|
|
||
46
|
|
|
|
|
Incorporated by Reference |
|
|||||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed Herewith |
||
|
|
2015 Stock Incentive Plan, as amended June 13, 2017.
|
|
8-K |
|
001-36833 |
|
10.1 |
|
09/12/17 |
|
|
|||
|
10.20(a)# |
|
Form of Notice of Stock Option Grant and Stock Option Agreement under the 2015 Stock Incentive Plan.
|
|
S-8 |
|
333-214118 |
|
10.2 |
|
10/14/16 |
|
|
||
|
10.20(b)# |
|
Form of Notice of Restricted Stock Award and Restricted Stock Agreement under the 2015 Stock Incentive Plan.
|
|
S-8 |
|
333-214118 |
|
10.3 |
|
10/14/16 |
|
|
||
|
10.20(c)# |
|
Form of Notice of Stock Bonus Award and Stock Bonus Award Agreement under the 2015 Stock Incentive Plan
|
|
S-8 |
|
333-214118 |
|
10.4 |
|
10/14/16 |
|
|
||
|
10.20(d)# |
|
Form of Notice of Stock Appreciation Right Award and Stock Appreciation Right Award Agreement under the 2015 Stock Incentive Plan.
|
|
S-8 |
|
333-214118 |
|
10.5 |
|
10/14/16 |
|
|
||
|
10.20(e)# |
|
Form of Notice of Restricted Stock Unit Award and Restricted Stock Unit Agreement under the 2015 Stock Incentive Plan.
|
|
S-8 |
|
333-214118 |
|
10.6 |
|
10/14/16 |
|
|
||
|
10.20(f)# |
|
Form of Notice of Performance Shares Award and Performance Shares Agreement under the 2015 Stock Incentive Plan.
|
|
S-8 |
|
333-214118 |
|
10.7 |
|
10/14/16 |
|
|
||
|
|
Faulkes Executive Chairman Agreement with VolitionRX dated March 31, 2015.
|
|
10-Q |
|
001-36833 |
|
10.32 |
|
5/12/15 |
|
|
|||
|
|
First Amendment to Executive Chairman’s Agreement between VolitionRX and Dr. Faulkes, dated May 11, 2016.
|
|
10-Q |
|
001-36833 |
|
10.1 |
|
5/13/16 |
|
|
|||
|
|
Independent Director Agreement.
|
|
10-Q |
|
001-36833 |
|
10.33 |
|
5/12/15 |
|
|
|||
|
|
Real Estate Capital Lease Agreement by and between Belgian Volition and ING Asset Finance Belgium S.A., dated October 4, 2016 (English translation of French original).
|
|
8-K |
|
001-36833 |
|
10.1 |
|
10/31/16 |
|
|
|||
|
|
Deed of Sale to the Sale Agreement by and between and Gerard Dekoninck S.A., dated October 25, 2016 (English translation of French original).
|
|
8-K |
|
001-36833 |
|
10.2 |
|
10/31/16 |
|
|
|||
|
|
Agreement by and between VolitionRX and Isosceles dated May 2, 2014.
|
|
S-1/A |
|
333-200628 |
|
10.30 |
|
1/23/15 |
|
|
|||
|
|
Employment Agreement by and between Volition Diagnostics UK Limited and Cameron Reynolds, dated March 7, 2017.
|
|
10-K |
|
001-36833 |
|
10.27 |
|
03/10/17 |
|
|
|||
|
|
Employment Agreement by and between Volition Diagnostics UK Limited and Jacob Micallef, dated March 7, 2017.
|
|
10-K |
|
001-36833 |
|
10.28 |
|
03/10/17 |
|
|
|||
47
|
|
|
|
|
Incorporated by Reference |
|
||||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed Herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employment Agreement by and between Volition Diagnostics UK Limited and Rodney Rootsaert, dated March 7, 2017.
|
|
10-K |
|
001-36833 |
|
10.29 |
|
03/10/17 |
|
|
||
|
|
Employment Agreement by and between Volition Diagnostics UK Limited and Martin Faulkes, dated March 7, 2017.
|
|
10-K |
|
001-36833 |
|
10.30 |
|
03/10/17 |
|
|
||
|
|
Employment Agreement by and between Volition Diagnostics UK Limited and David Vanston, dated April 10, 2017.
|
|
10-Q |
|
001-36833 |
|
10.1 |
|
05/11/17 |
|
|
||
|
|
Unsecured Credit Agreement, dated September 20, 2017, by and among VolitionRX Limited, Belgian Volition SPRL and SOFINEX (English translation of French original).
|
|
8-K |
|
001-36833 |
|
10.1 |
|
09/21/17 |
|
|
||
|
|
Clinical Study Agreement, dated July 17, 2017, by and between Volition America, Inc. and the Regents of the University of Michigan
|
|
10-Q |
|
001-36833 |
|
10.1 |
|
11/09/17 |
|
|
||
|
|
List of Subsidiaries.
|
|
|
|
|
|
|
|
|
|
X |
||
|
|
Consent of independent registered public accounting firm.
|
|
|
|
|
|
|
|
|
|
X |
||
|
24.1 |
|
Power of Attorney (included on the signature page of this report).
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
Certification of Chief Executive Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) promulgated under the Securities Exchange Act of 1934, as amended.
|
|
|
|
|
|
|
|
|
|
X |
||
|
|
Certification of Chief Financial Officer pursuant to Rule 13a-14(a) or Rule 15d-14(a) promulgated under the Securities Exchange Act of 1934, as amended.
|
|
|
|
|
|
|
|
|
|
X |
||
|
32.1* |
|
Certifications of Chief Executive Officer and Chief Financial Officer, pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002.
|
|
|
|
|
|
|
|
|
|
X |
|
|
10.1 INS |
|
XBRL Instance Document.
|
|
|
|
|
|
|
|
|
|
X |
|
|
101.SCH |
|
XBRL Taxonomy Extension Schema Document.
|
|
|
|
|
|
|
|
|
|
X |
|
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase Document.
|
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
48
|
|
|
|
|
Incorporated by Reference |
|
|
||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed Herewith |
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document.
|
|
|
|
|
|
|
|
|
|
X |
|
101.LAB |
|
XBRL Taxonomy Extension Label Linkbase Document.
|
|
|
|
|
|
|
|
|
|
X |
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase Document. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
# |
|
Indicates a management contract or compensatory plan or arrangement.
|
||||||||||
|
* |
|
The certifications attached as Exhibit 32.1 accompany this report pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, and shall not be deemed “filed” by the registrant for purposes of Section 18 of the Exchange Act and are not to be incorporated by reference into any of the registrant’s filings under the Securities Act or the Exchange Act, irrespective of any general incorporation language contained in any such filing. |
||||||||||
ITEM 16.FORM 10-K SUMMARY
None.
49
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant caused this report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
|
|
|
|
VOLITIONRX LIMITED |
|
|
|
|
|
|
|
|
|
|
|
|
|
Dated: March 1, 2018 |
|
|
|
By: /s/ Cameron Reynolds |
|
|
|
|
|
Cameron Reynolds |
|
|
|
|
|
President, Chief Executive Officer and Director |
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each individual whose signature appears below constitutes and appoints Cameron Reynolds and Rodney Rootsaert, and each or either of them, acting individually, his or her true and lawful attorney-in-fact and agent, with full power of substitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this report on Form 10-K, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or either of them, or his, her or their substitute or substitutes, may lawfully do or cause to be done or by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this report on Form 10-K has been signed below by the following persons in the capacities and on the date indicated.
|
Signature |
Title |
Date |
|
|
|
|
|
/s/ Cameron Reynolds Cameron Reynolds |
President, Chief Executive Officer and Director (Principal Executive Officer) |
March 1, 2018 |
|
|
|
|
|
/s/ David Vanston David Vanston |
Chief Financial Officer and Treasurer (Principal Financial and Accounting Officer) |
March 1, 2018 |
|
|
|
|
|
/s/ Dr. Martin Faulkes Dr. Martin Faulkes |
Director |
March 1, 2018 |
|
|
|
|
|
/s/ Guy Innes Guy Innes |
Director |
March 1, 2018 |
|
|
|
|
|
/s/ Dr. Alan Colman Dr. Alan Colman |
Director |
March 1, 2018 |
|
|
|
|
|
/s/ Dr. Habib Skaff Dr. Habib Skaff |
Director |
March 1, 2018 |
|
|
|
|
|
/s/ Dr. Edward Futcher Dr. Edward Futcher |
Director |
March 1, 2018 |
|
|
|
|
50