10-K: Annual report pursuant to Section 13 and 15(d)
Published on March 30, 2022
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM
ANNUAL REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the Fiscal Year Ended
TRANSITION REPORT PURSUANT TO SECTION 13 OR 15(d) OF THE SECURITIES EXCHANGE ACT OF 1934 |
For the transition period from __________ to __________
Commission File Number:
(Exact name of registrant as specified in its charter) |
|
||
|
(State or other jurisdiction of incorporation or organization) |
|
(I.R.S. Employer Identification No.) |
(Address of principal executive offices)
+1 (
(Registrant’s telephone number, including area code)
Securities registered pursuant to Section 12(b) of the Act:
Title of Each Class: |
|
Trading Symbol(s) |
|
Name of Each Exchange on Which Registered: |
|
|
Securities registered pursuant to Section 12(g) of the Act: None
Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐
Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or Section 15(d) of the Act. Yes ☐
Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports) and (2) has been subject to such filing requirements for the past 90 days.
Indicate by check mark whether the registrant has submitted electronically every Interactive Data File required to be submitted pursuant to Rule 405 of Regulation S-T (§ 232.405 of this chapter) during the preceding 12 months (or for such shorter period that the registrant was required to submit such files).
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, a smaller reporting company, or an emerging growth company. See the definitions of “large accelerated filer,” “accelerated filer,” “smaller reporting company” and “emerging growth company” in Rule 12b-2 of the Exchange Act:
Large accelerated filer |
☐ |
Accelerated filer |
☐ |
☒ |
Smaller reporting company |
||
|
|
Emerging growth company |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
Indicate by check mark whether the registrant has filed a report on and attestation to its management’s assessment of the effectiveness of its internal control over financial reporting under Section 404(b) of the Sarbanes-Oxley Act (15 U.S.C. 7262(b)) by the registered public accounting firm that prepared or issued its audit report.
Indicate by check mark whether the registrant is a shell company (as defined in Rule 12b-2 of the Act). Yes
As of June 30, 2021, the last trading day of the registrant’s most recently completed second fiscal quarter, the aggregate market value of the voting common stock held by non-affiliates of the registrant was $
As of March 25, 2022, there were
Documents incorporated by reference:
Portions of the registrant’s Proxy Statement for its 2022 Annual Meeting of Stockholders, to be filed on or before May 2, 2022 are incorporated by reference into Part III, Items 10-14 of this Annual Report on Form 10-K.
Table of Contents
| Table of Contents |
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This Annual Report on Form 10-K for the fiscal year ended December 31, 2021, (this “Report”), and the information and documents incorporated by reference in this Report, contain “forward-looking statements” within the meaning of Section 27A of the Securities Act of 1933, as amended (the “Securities Act”), and Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), which statements are subject to considerable risks and uncertainties. These forward-looking statements are intended to qualify for the safe harbor from liability established by the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical fact included in this Report or incorporated by reference into this Report are forward-looking statements. We have attempted to identify forward-looking statements by using words such as “aim,” “anticipate,” “believe,” “continue,” “could,” “estimate(s),” “expect,” “forecast(s),” “goal,” “intend,” “may,” “plan(s),” “potential,” “project,” “seek,” “should,” “strategy,” “will,” and other forms of these words or similar words or expressions or the negative thereof (although not all forward-looking statements contain these words). In particular, forward-looking statements contained in this Report, and the information and documents incorporated by reference within this Report, relate to, among other things, our predictions of earnings, revenues, expenses or other financial items; plans or expectations with respect to our development activities or business strategy, including regulatory approvals, commercialization and market acceptance; statements concerning industry trends and industry size; statements regarding anticipated demand for our products and market opportunity, or the products of our competitors; statements relating to manufacturing forecasts, and the potential impact of our relationship with contract manufacturers and original equipment manufacturers on our business; assumptions regarding the future cost and potential benefits of our research and development efforts; the effect of critical accounting policies; forecasts of our liquidity position or available cash resources; and statements relating to the assumptions underlying any of the foregoing. We caution you that the foregoing list may not include all of the forward-looking statements made in this Report and the information and documents incorporated by reference within this Report.
We have based our forward-looking statements on our current assumptions, expectations and projections about trends affecting our business and industry and other future events. Although we do not make forward-looking statements unless we believe we have a reasonable basis for doing so, we cannot guarantee their accuracy. Forward-looking statements are subject to substantial known and unknown risks and uncertainties that could cause our future business, financial condition, results of operations or performance to differ materially from our historical results or those expressed or implied in any forward-looking statement contained in this Report.
Some significant factors that may impact our estimates and forward-looking statements include, but are not limited to:
|
· |
Our inability to generate any significant revenue or achieve profitability; |
|
· |
Our need to raise additional capital in the future; |
|
· |
Our expectations to expand our product development, research and sales and marketing capabilities could give rise to difficulties in managing our growth; |
|
· |
Our limited experience with direct sales and marketing; |
|
· |
The material weaknesses in our internal control over financial reporting that we have identified; |
|
· |
The possibility that we may not be able to continue to operate, as indicated by the “going concern” opinion from our auditors; |
|
· |
Our ability to successfully develop, manufacture, market, and sell our future products; |
|
· |
Our ability to timely obtain necessary regulatory clearances or approvals to distribute and market our future products; |
|
· |
The acceptance by the marketplace of our future products; |
|
· |
The highly competitive and rapidly changing nature of the cancer diagnostics market; |
|
· |
Our reliance on third parties to manufacture and supply our intended products, and such manufacturers’ dependence on third party suppliers; |
|
· |
Our dependence on third party distributors; |
|
· |
Protection of our patents, intellectual property and trade secrets; and |
|
· |
Business disruptions and economic and other uncertainties surrounding the COVID-19 pandemic. |
For additional information, refer to the section entitled “Risk Factors” in Part I, Item 1A of this Report, and the other documents that we have filed with the U.S. Securities and Exchange Commission (the “SEC”).
In addition, actual results may differ as a result of additional risks and uncertainties of which we are currently unaware or which we do not currently view as material to our business. For these reasons, readers are cautioned not to place undue reliance on any forward-looking statements.
| 1 |
| Table of Contents |
You should read this Report in its entirety, including the documents that we file as exhibits to this Report and the documents we incorporate by reference into this Report, with the understanding that our future results may be materially different from what we currently expect. The forward-looking statements we make speak only as of the date on which they are made. We expressly disclaim any intent or obligation to update any forward-looking statements after the date hereof to conform such statements to actual results or to changes in our opinions or expectations. If we do update or correct any forward-looking statements, readers should not conclude that we will make additional updates or corrections.
Use of Terms
Except as otherwise indicated by the context, references in this Report to “Company,” “VolitionRx,” “Volition,” “we,” “us,” and “our” are references to VolitionRx Limited and its wholly owned subsidiaries, Singapore Volition Pte. Limited, Belgian Volition SRL, Volition Diagnostics UK Limited, Volition Germany GmbH, Volition America, Inc, and Volition Global Services SRL, as well as majority owned subsidiary Volition Veterinary Diagnostics Development LLC. Additionally, unless otherwise specified, all references to “$” refer to the legal currency of the United States of America.
NucleosomicsTM and Nu.Q® and their respective logos are trademarks and/or service marks of VolitionRx and its subsidiaries. All other trademarks, service marks and trade names referred to in this Report are the property of their respective owners.
| 2 |
| Table of Contents |
PART I
ITEM 1. |
BUSINESS |
Overview
Volition is a multi-national epigenetics company that applies its Nucleosomics™ platform through its subsidiaries to develop simple, easy to use, cost-effective blood tests to help diagnose and monitor a range of life-altering diseases including certain cancers and diseases associated with NETosis such as sepsis and COVID-19. Our mission is to save lives and improve outcomes for millions of people and animals worldwide. Early diagnosis and monitoring have the potential to not only prolong the life of patients, but also to improve their quality of life.
Our tests are based on the science of Nucleosomics™, which is the practice of identifying and measuring nucleosomes in the bloodstream or other bodily fluid - an indication that disease is present. We are primarily focused on human diagnostics and monitoring but also have a subsidiary focused on animal diagnostics and monitoring.
We have five key pillars of focus: Nu.Q®, Nu.Q® NETs, Nu.Q® Capture, Nu.Q® Discover and Nu.Q® Vet, all of which use the same proprietary Nu.Q® platform to commercialize in different areas.
Our research and development activities are centered in Belgium, with an innovation laboratory in California, and additional offices in Texas, London, and Singapore, where we focus on bringing our diagnostic and disease monitoring products to market.
Volition’s Solution and the Science Behind It
We are dedicated to revolutionizing the diagnosis and monitoring of life-altering diseases by advancing the science of epigenetics. Imagine a world where diseases like cancer and sepsis can be diagnosed early and monitored easily using routine blood tests. That’s the world we’re trying to build by developing our innovative family of simple, easy to use, cost-effective Nu.Q® tests.
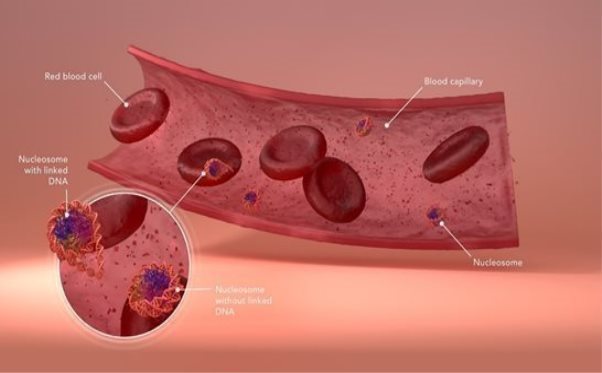
Our patented Nucleosomics™ technology uses chromosomal structures called nucleosomes as biomarkers in cancer and other diseases: as explained below, chromosomes consist of the genetic material (DNA) wrapped in a coat of proteins and other molecules. All the tests in our portfolio detect various characteristic changes in nucleosomes that occur from the earliest stages of disease, enabling early detection and potentially a better way to monitor disease progression and the patient’s response to treatment.
Unlocking Epigenetics
We believe epigenetics is the most exciting field in disease detection and management today. Modern genetics - the study of genes and heredity, is underpinned by the linear sequences of molecular “letters” present in the DNA double helix of each living cell, many of which encode the genes. It has had an enormous impact on the practice of medicine, revolutionizing the way doctors identify people with inherited conditions, diagnose cancer, and, increasingly, design personalized treatment plans. However, there’s more to chromosomes than just the DNA sequence; at Volition, we focus on chromosomes’ second epigenetic code, which contains a wealth of additional information about the health and function of the body’s cells. You can think of the DNA sequence of each cell as the text of an instruction manual, and epigenetics as the formatting. Some parts of the manual are bolded, highlighted, or underlined, telling the cell to emphasize those sections, while others are struck out, telling the cell to ignore those genes.
The cells of most bodily organs are continuously replaced by new ones. As they die, many old cells release their nucleosomes into the bloodstream. Our patented Nucleosomics™ technology isolates these circulating nucleosomes from the blood for quantification and analysis.
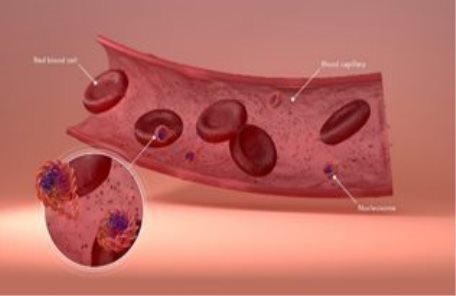
| 3 |
| Table of Contents |
Chromosome and nucleosome structure represent a major mechanism for epigenetic control. Each chromosome contains one long, single molecule of DNA that is coated by a complex array of proteins, mostly in the form of nucleosomes, giving the stretched-out, unwound DNA/protein core, or chromatin, the appearance of “beads on a string.” Unwound chromatin is accessible for reading (or transcribing), and unwound genes may be active. However, genes with coiled or supercoiled nucleosomes are inaccessible and inactive.
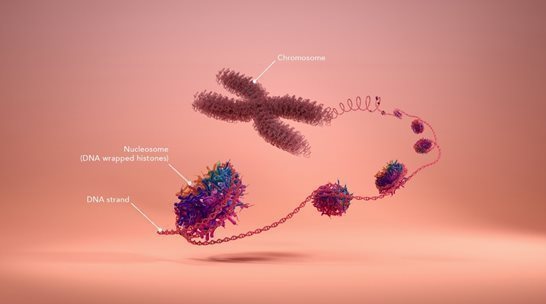
Each nucleosome consists of a disc of eight histone proteins wrapped by a short length of DNA. Nucleosome structure has a dual role: first, it allows the compact storage and protection of the genetic material (or DNA), and second, it modulates the epigenetic regulation (transcription) of that DNA. This regulation is achieved through reversible chemical changes to both the DNA and protein components as well as through the binding of specific regulatory proteins to the DNA.
Volition’s Epigenetic Approach
Through our Nu.Q® (short for nucleosome quantification) family of tests in our five key pillars, we aim to offer a new, convenient and cost-effective approach to the detection, diagnosis and monitoring of diverse diseases from a simple blood test.
Highlighting abnormalities
Our technology seeks to detect characteristic epigenetic changes in nucleosomes that occur from the earliest stages of cancer and other diseases. Epigenetic changes often occur before the diseased cells themselves become abnormal enough to show up in traditional biopsies, and oftentimes before the first symptoms are felt. We aim to replace unpleasant, invasive, and often expensive screening and diagnostic tests such as colonoscopies and biopsies with Nu.Q® blood tests, helping to save lives and to reduce overall healthcare costs.
Population screening
Our technology and tests could potentially play a game-changing role in early detection of disease in asymptomatic people via routine, population-wide screening. We believe that simple, cost-effective, and accurate tests are the “holy grail” of an effective screening program.
Risk stratification and diagnostic aid
In addition to being highly informative in their own right, Nu.Q® tests have the potential to improve the sensitivity and specificity of other clinical tests. Our tests could also reduce the number of people needing invasive biopsies and other diagnostic procedures, which can be expensive and harmful.
| 4 |
| Table of Contents |
Disease and treatment monitoring
Nu.Q® tests may act as an early warning system by monitoring treatment response, disease progression and remission.
Improving Outcomes for Patients
We have five key pillars of focus: Nu.Q®, Nu.Q® NETs, Nu.Q® Capture, Nu.Q® Discover and Nu.Q® Vet, all using the same proprietary Nu.Q® platform.
Nu.Q® - Detecting cancer early to save lives.
We are developing a simple, cost-effective blood test for cancer. Cancer is a devastating disease that touches many peoples’ lives, accounting for approximately 10 million deaths worldwide each year. Early diagnosis is the best way to improve someone’s chances of surviving cancer; however, current population-wide screening tests (such as mammograms and colonoscopies) are often invasive and unpleasant. They can also be expensive, causing many people to miss routine screening. There are no population screening tests at all for some types of cancer, including aggressive forms of the disease such as ovarian or pancreatic cancers. Unfortunately, many patients are therefore diagnosed too late, when their cancer has already spread, and treatment is more difficult. We believe that Nu.Q® can become a cost-effective routine blood test for multiple types of cancer, allowing doctors to check off an extra box along with other routine blood tests like cholesterol during an annual wellness visit. Nu.Q® tests have further potential applications in clinical oncology beyond cancer detection. Being able to use epigenetic information from tumor cells’ nucleosomes could also help physicians select the best treatment for each patient, monitor their response and the disease progression.
We are currently investigating the potential use of Nu.Q® tests in a range of cancers and clinical settings including:
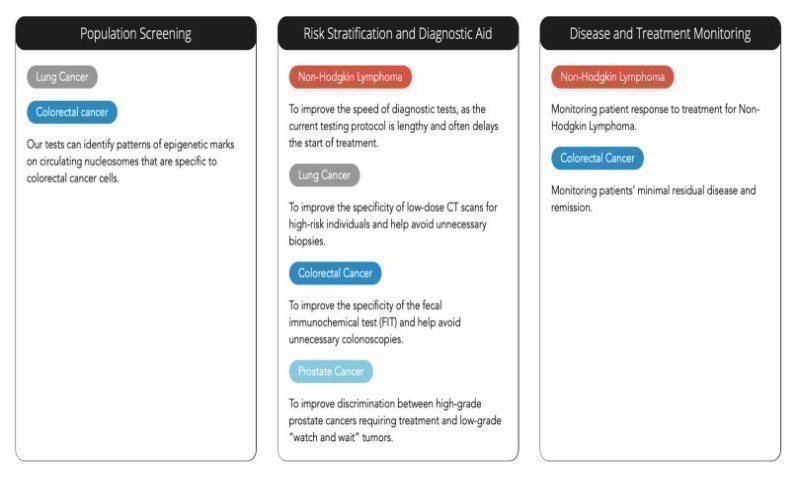
| 5 |
| Table of Contents |
Nu.Q® NETs - Monitoring the immune system to save lives.
The immune system can be both friend and foe; a potent protective force that sometimes overreacts, damaging the body’s own cells and tissues in the process. We are working to develop tests that will identify people at high risk of poor outcomes/death caused by an immune system overreaction to COVID-19 and other infections. The immune system is comprised of many different types of white blood cells with different functions. The most abundant of these white blood cells are neutrophils, which serve as a first line of defense. When neutrophils detect bacteria, viruses, injuries, or other threats, these cells produce Neutrophil Extracellular Traps (“NETs”), which are sticky webs made of long strings of nucleosomes that work to inhibit a perceived threat from spreading through the body.
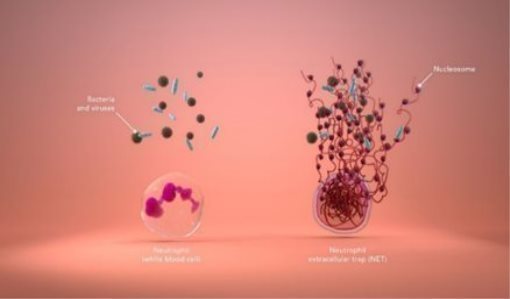
Although NETs are an important part of the body’s response to infection, the presence of too many of them in the blood can tip the immune system’s delicate balance between reaction and overreaction. Elevated levels of NETs are a complicating factor associated with poor patient outcomes in a range of infectious and non-infectious diseases.
Sepsis—widespread tissue and organ damage triggered by an abnormal immune response to an infection—is an area of particular focus for our research on NETs. A recent global study estimated that there were approximately 49 million cases and 11 million sepsis-related deaths worldwide in 2017, accounting for approximately 20% of all deaths from the same year.
Severe cases of COVID-19 can cause excessive production of NETs in the lungs, which can lead to severe lung impairment or death. Because NETs contain nucleosomes, our proprietary Nu.Q® nucleosome assays have been shown to detect NETs. Using our Nu.Q® nucleosome assays could enable the stratification of patients with a high level of NETs, allow physicians to rapidly triage these patients, and monitor their disease progression and response to treatment.
| 6 |
| Table of Contents |
The focus on sepsis due to the COVID-19 pandemic has accelerated our research on NETs. Our current programs include:
Nu.Q® Capture - Capturing and concentrating samples for more accurate diagnosis - Locating the needle in a haystack.
Human blood is a mixture of many different cell types floating in a complex soup of proteins and other molecules, including nucleosomes released by cells from all around the body. Detecting a handful of cancerous or other abnormal cells in a patient’s blood sample has historically been like finding a proverbial needle in a haystack. Volition’s Nu.Q® Capture program has several strands of technology which either essentially removes background noise, thereby amplifying the signal or looks to identify the signal in a novel way. This sample enrichment tool removes healthy nucleosomes, leaving an enriched sample of abnormal nucleosomes behind for further analysis. These nucleosomes contain tumor-specific DNA “typos,” epigenetic changes, and other biomarkers that when analyzed could potentially be used to diagnose a specific type of cancer or other medical condition, guide treatment selection, and monitor disease and treatment progress. Other strands of Nu.Q® Capture technology involve isolating various chromatin fragments including nucleosomes and transcription factors from plasma for analysis by mass spectrometry and next-generation DNA sequencing.
Deploying Nu.Q® Capture as the first blood sample processing step could potentially:
|
· |
Enhance the sensitivity of subsequent Nu.Q® immunoassays for diagnosing and monitoring different types of disease using our proprietary Nucleosomics™ platform. |
|
· |
Aid the development of improved diagnostic DNA sequencing methods. |
|
· |
Serve as a quality control tool to reduce the rate of clinical test failure, saving time that is especially valuable for people whose test results are being used to inform their treatment. |
|
· |
Aid the discovery of new biomarkers. |
|
· |
Allow the complete profiling of nucleosomes. |
Another novel method utilizing Nu.Q® Capture and mass spectrometry was published in 2021 and demonstrated the detection and quantification of histone modifications present in the circulating nucleosomes in the blood of cancer patients. We believe that our work has highlighted for the first time that histone H2A1R3 citrulline is, in plasma, upregulated in colorectal cancer patients and so could be a biomarker we target for future Nu.Q® immunoassay development. Furthermore, the use of Nu.Q® Capture may open up the possibility of using mass spectrometry not only for biomarker discovery as demonstrated in this publication but also as a high throughput platform for screening and/or diagnostics when used in combination with either sequencing and/or our Nu.Q® assays.
| 7 |
| Table of Contents |
This technology sheds new light on epigenetic changes that cannot be effectively detected amid the noise left behind when using current testing methods, leading to better clinical tests and potentially improved outcomes in the future. Volition is engaged in multiple research collaborations with academic laboratories working at the cutting edge of their respective fields, to ensure we take advantage of the latest findings and turn them into new clinical tools as quickly as possible.
Nu.Q® Discover - A complete solution to profiling nucleosomes.
Nu.Q® Discover gives clinicians, patients, and researchers access to a range of state-of-the-art assays, built on our proprietary Nucleosomics™ platform, for rapid epigenetic profiling in disease model development, preclinical testing and clinical trials. Our H3.1 assay is also available for purchase as a Research Use Only kit. Our assays run in our Silver One facility in Belgium or on site and can be used to answer clinical questions, such as measuring treatment efficacy, or on-target and off-target effects in drug development. Applications include biomarker discovery in oncology, inflammatory conditions, diabetes and more. Existing and potential customers include both pharmaceutical companies and academic research institutions.
Nu.Q® Vet (through Volition Vet)
Cancer is the most common cause of death in dogs over the age of two years in the United States. Up to 50% of all dogs over the age of 10 will develop cancer in their lifetimes. With approximately 77 million pet dogs in the United States, there are an estimated six million pet dogs diagnosed with cancer each year. As with humans, earlier detection can save lives and can also improve the quality of life of the dog and its owner. Yet, as of today, there are few single assay cancer blood tests on the veterinary market. Currently, dogs suspected of having cancer are required to undergo a variety of diagnostic tests that may be expensive, time consuming, and painful for the animal. We hope to change this with the introduction of the Nu.Q® Vet Cancer Screening Test: a simple, cost-effective, easy to use enzyme-linked immunosorbent assay (“ELISA”) based screening blood test which may help streamline the diagnostic process for older or “at-risk” dogs.
Data have been published in peer-reviewed journals demonstrating Nu.Q® Vet’s detection of common canine cancers such as lymphoma and hemangiosarcoma. More recently, data have been presented at the Veterinary Cancer Society Annual Conference suggesting that Nu.Q® Vet may also serve as a more sensitive measurement of both minimal residual disease and remission and could be a useful monitoring test for dogs with cancer.
We are currently conducting ongoing research regarding Nu.Q® Vet as follows:
Broadening the Range of Cancer Detected
|
· |
We have conducted work in other canine cancers and anticipate a further peer reviewed publication in 2022. |
|
· |
Although thus far the Nu.Q® Vet Cancer Screening Test has been marketed as a screening test for lymphoma and hemangiosarcoma, this Test may be useful in detecting other forms of cancer as well. |
|
· |
The Nu.Q® Vet Cancer Screening Test performs best for tumors that are more systemic (higher metastatic rate) or have a high cellular turnover rate. |
|
· |
We are also working to incorporate additional histone modifications into the Nu.Q® Vet Cancer Screening Test that will help to better differentiate between various cancer types. |
| 8 |
| Table of Contents |
Differential Diagnosis
|
· |
We are currently developing additional assays to add to the Nu.Q® Vet Cancer Screening Test to better differentiate inflammatory and other conditions from cancer. |
|
|
· |
Studies are underway at five U.S. university hospitals to collect data comparing a variety of concomitant conditions including: |
|
|
|
|
|
|
· |
Inflammatory conditions |
|
· |
Immune mediated disease |
|
· |
Endocrinopathies |
Point of Care Test
|
· |
We are in the process of developing a point of care test to aid the timely provision of diagnosis and treatment response. |
Over the next 12 to 24 months, we are planning to explore and evaluate the potential use and early detection efficacy of our Nu.Q® Vet platform in cats (related to cancer), horses (related to disease and performance fitness), and cattle (for feedlot disease).
Manufacturing Capabilities and Strategy
Our Silver One site in Belgium offers cutting edge, purpose-built manufacturing and processing facilities. We currently manufacture our own plates and large-scale manufacturing of our antibodies on beads. Our expert team is on hand throughout, to offer guidance and support and to fulfil customer needs. Our objective is to establish long-term mutually beneficial commercial relationships.
Commercialization Strategy
We believe, given the global prevalence of cancer and diseases associated with NETosis, and the low-cost, accessible and routine nature of our tests, Nu.Q® could potentially be used throughout the world.
We have developed and are continuing to develop a large portfolio of intellectual property (“IP”), centered around the science of identifying and measuring nucleosomes in the bloodstream. We call this science Nucleosomics™. Our technologies have a large range of applications, both in humans and animals, to screen, diagnose, and risk stratify patients, and to monitor treatments, disease progression and potential remissions. While we initially focused on cancer, we have now broadened the range of indications to include several diseases associated with NETosis, including sepsis, which is estimated to be responsible for one in five deaths worldwide.
Our launch sequence is largely determined by the regulatory hurdles we face; consequently, we aim to initially launch in Europe and Asia, and subsequently in the United States.
We aim to remain an IP powerhouse in the Nucleosomics™ space and expect to monetize our IP and technologies through licensing and distribution contracts with companies with established distribution networks on a worldwide or regional basis, in both human and animal care.
The first series of products we expect to launch, following the roll-out of our canine cancer screening test, are:
|
· |
a canine cancer monitoring test; |
|
· |
a NETosis related screening and monitoring test; |
|
· |
Nu.Q® Discover; our biomarkers for research purposes and to support clinical trials; and |
|
· |
Cancer tests for humans in Non-Hodgkin's Lymphoma, colorectal cancer and lung. |
Our Nucleosomics™ technology is transferable to multiple platforms such as ELISA 96-well plates, bead-based chemiluminescent and we are currently working on transferring our technology to the widely-utilized homogeneous immunoassay or HIA platform and several point of care platforms to enable rapid turnaround of results in clinic/the doctor’s office.
Additionally, we are working on complete nucleosome analysis with our Nu.Q® Capture technology. The goal of this project is to investigate ways to specifically target circulating tumor DNA (“ctDNA”). The ability to enrich ctDNA will allow us to use mass spectrometry to analyze histone and DNA modifications, and moreover to sequence the DNA present around the nucleosomes. This information might enable cancer diagnosis to identify the tissue of origin of that given cancer.
| 9 |
| Table of Contents |
Our Market Opportunity
Volition applies its Nucleosomics™ platform through its subsidiaries to develop simple, easy to use, cost-effective blood tests to help diagnose and monitor a range of life-altering diseases for both humans and animals including certain cancers and diseases associated with NETosis such as sepsis and COVID-19. Given the wide-ranging nature of our products in development we believe that our market opportunity is large.
Based on our calculations, we believe that Volition’s annual total addressable market is approximately $70 billion. Key assumptions for this market forecast are:
|
· |
Nu.Q® Vet: opportunity is calculated based on canine and feline populations that are eligible for screening and monitoring. |
|
· |
Nu.Q® Discover: opportunity is calculated using drug pipeline data (registered clinical trial programs) for relevant epigenetic targets. |
|
· |
Nu.Q® NETs: opportunity is calculated based on average length of stay and estimated hospital admissions and discharges for sepsis. |
|
· |
Nu.Q® Cancer: opportunity is calculated based on eligible population for annual screening, target participation rates and incidence/prevalence of specific cancers and risk stratification use cases. |
We have assumed the following prices per test:
|
· |
Human: $120 for the U.S., €45 for Europe, $25 for the rest of the world. |
|
· |
Veterinary: $50 globally. |
To the extent that one or more of our assumptions prove incorrect our calculation could be materially impacted.
We anticipate that because of their ease of use and cost efficiency of our tests they have the potential to become the first method of choice for disease detection and monitoring in both humans and animals.
Our Competition
We anticipate facing competition primarily from other human focused healthcare, pharmaceutical and diagnostic companies such as Exact Sciences Corporation, Guardant Health, GRAIL Inc., Freenome Holdings Inc, CellMax Life, Archer DX Inc., Foundation Medicine Inc., Oncocyte Corporation, OpKo Health Inc., MDNA Life Sciences Inc., Oncimmune Holdings Plc, Abbott Laboratories Inc., Cepheid Inc., Koninklijke Philips N.V., GE Healthcare, Siemens, Gen-Probe Incorporated, EpiGenomics AG, MDxHealth SA, and Roche Diagnostics, and from companies such as Mars Incorporated, IDEXX Laboratories Inc., PetDx, One Health Company (Fidocure) and Vidium Animal Health focused on the veterinary space. There may also be other companies developing products competitive with ours of which we are unaware.
We predict our future products will have a competitive edge compared to those offered by competitors on the basis that our tests are developed to be accurate, cost-effective, attractive from a government reimbursement perspective, easy to use, non-invasive, technologically advanced, and compatible with immunoassay systems, based on strong intellectual property and to be used for mass screenings.
Many of our competitors have substantially greater financial, technical, and other resources and larger, more established marketing, sales and distribution systems than we have. Many of our competitors also offer broad product lines outside of the diagnostic testing market and have brand recognition. Moreover, our competitors may make rapid technological developments that may result in our intended technologies and products becoming obsolete before we are able to enter the market, recover the expenses incurred to develop them or generate significant revenue. Our success will depend, in part, on our ability to develop our intended products in a timely manner, keep our future products current with advancing technologies, achieve market acceptance of our future products, gain name recognition and a positive reputation in the healthcare industry, and establish successful marketing, sales and distribution efforts.
Government Regulations
The healthcare industry, and thus our business, is subject to extensive federal, state, local and foreign regulation. Some of the pertinent laws have not been definitively interpreted by the regulatory authorities or the courts, and their provisions are open to a variety of subjective interpretations. In addition, these laws and their interpretations are subject to change.
Both United States federal and state governmental agencies continue to subject the healthcare industry to intense regulatory scrutiny, including heightened civil and criminal enforcement efforts. As indicated by work plans and reports issued by these agencies, the federal government will continue to scrutinize, among other things, the marketing, labeling, promotion, manufacturing, and export of diagnostic healthcare products. The federal government also has increased funding in recent years to fight healthcare fraud, and various agencies, such as the United States Department of Justice, the Office of Inspector General of the Department of Health and Human Services, and state Medicaid fraud control units, are coordinating their enforcement efforts.
| 10 |
| Table of Contents |
Commercialization of our future products in the clinical IVD market (e.g. for patient diagnosis in hospitals, clinics, etc.) requires government approval (CE marking in Europe, FDA approval in the United States, and Chinese Food and Drug Administration (“CFDA”) approval in China). Our diagnostic products fall within the IVD medical device category and are subject to FDA clearance or approval in the United States. We anticipate our tests will have to be cleared through the FDA’s premarket notification (“510(k)”), process, or its premarket approval (“PMA”) process. The determination of whether a 510(k) or a PMA is necessary will depend in part on the proposed indications for use and the FDA’s assessment of the risk associated with the use of the IVD for a particular indication. A similar system operates in China through the CFDA.
In Europe, IVD medical devices are regulated by the European Directive 98/79/EC (“EU IVDD”), where products not listed in ANNEX II, such as the ones developed by Volition, can be CE marked through a self-certification process. Under this system, manufacturers must operate a Quality System and build/maintain a technical documentation file demonstrating the conformity of the product with the requirements of the EU IVDD. This includes the validation of the devices in a limited clinical trial to demonstrate the manufacturer has met analytical and clinical performance criteria. The manufacturer then issues a declaration of conformity and affixes the CE mark logo to the product.
In May 2017, the new European In Vitro Diagnostic Regulation 2017/746 (“EU IVDR”), became effective, marking the start of a transition period for manufacturers selling IVD devices into Europe. The date of application of the EU IVDR, which replaces the EU IVDD, is May 26, 2022. After this date, no new applications pursuant to the former EU IVDD will be accepted. We believe the most challenging changes under the IVDR will be those regarding the classification of products, which will bring almost all IVDs under the direct review and control of designated assessment organizations (“Notified Bodies”), and the performance evaluation of IVDs, which will require extensive clinical and analytical performance studies in addition to a demonstration of scientific validity. Additional requirements will be applied to reinforce the safety of the products such as extended responsibilities of the economic actors of the supply chain, increased post marketing surveillance activities, unannounced audits from Notified Bodies, implementation of an improved traceability and transparency of the devices with the introduction of the Unique Device Identification system and an expanded European Database on Medical Devices.
In January 2022, the EU IVDR was amended. The May 26, 2022, date of application of the EU IVDR remains unchanged. Tailored transitional periods have, however, been introduced for devices that must undergo a conformity assessment involving Notified Bodies for the first time under the EU IVDR. The length of the transitional periods depends on the classification of device.
In practice, the conformity assessment procedure for our products will be a combination of Quality Management System (“QMS”) audits and Technical Documentation assessments. The time needed for a Technical Documentation assessment of a device by our Notified Body (“TÜV SÜD”) is expected to last for nine months at a minimum. We have already begun discussions with the TÜV SÜD to ensure compliance with the EU IVDR as soon as possible.
To support the conformity to both the EU IVDD and the new IVDR, Belgian Volition has implemented a QMS, conforming to the internationally agreed standard ISO 13485 that sets out the QMS requirements specific to the medical devices industry. Belgian Volition has maintained its ISO certification since 2015.
We will also be required to comply with numerous other federal, state, and local laws relating to matters such as safe working conditions, industrial safety, and labor laws. We may incur significant costs to comply with such laws and regulations in the future, and lack of compliance could have material adverse effects on our operations.
We believe we have structured our business operations to comply with applicable legal requirements. However, it is possible that governmental entities or other third parties could interpret these laws differently and assert otherwise, which could have a material adverse impact on our business.
Intellectual Property
Volition is developing clinical products based on the enrichment and analysis of epigenetically modified circulating nucleosomes using immunoassay, mass spectrometry, DNA sequencing and other methods. We have used this position to build a growing, broad and strong patent portfolio covering the ability to profile the epigenetic environment surrounding circulating chromosome fragments from diseased cells, including the epigenetic signaling status of nucleosomes, DNA, and other epigenetic chromatin proteins.
| 11 |
| Table of Contents |
Our patent portfolio includes 29 patent families and a total 84 patents granted related to our diagnostic tests (including veterinary applications), with 12 patents granted in the United States, 14 patents granted in Europe, and a further 58 patents granted worldwide. Additionally, we have a total of 93 patent applications currently pending, worldwide.
We intend to continue our development of the Nucleosomics™ technologies and will continue to apply for patents for future product developments. Our IP strategy is to protect the technologies and gain market exclusivity with patents in Europe and the United States and in other strategic countries. The patents on the technologies underlying our products should provide broad coverage for each product, including protection through at least 2031.
Employees
As of December 31, 2021, we had 83 full-time equivalent (“FTE”) compared to 60 as of December 31, 2020. We continually assess employee turnover, recruitment initiatives, compensation and benefits programs, safety in performing critical laboratory work, diversity and other matters relevant to human capital management, and we review results with our board of directors on a periodic basis. We aim to offer competitive compensation (including salary, incentive bonus, and equity) and benefits packages in each of our locations and in each of our employee groups at each level around the globe as assessed with internal and external benchmarking data. We aim to build a pipeline for talent to create more opportunities for workplace diversity and to support greater representation within the Company.
Corporate History
VolitionRx Limited was originally incorporated on September 24, 1998 in the State of Delaware under the name “Standard Capital Corporation.” VolitionRx acquired its wholly owned operating subsidiary, Singapore Volition Pte. Limited, a Singapore registered company (“Singapore Volition”) in October 2011. Volition Global Services SRL, a Belgium private limited liability company (“Volition Global”), was formed in August 2021, which is a wholly owned operating subsidiary of VolitionRx. Singapore Volition has one subsidiary, Belgian Volition SRL, a Belgium private limited liability company (“Belgian Volition”), which it acquired in September 2010. Belgian Volition has four subsidiaries, Volition Diagnostics UK Limited, a private limited company formed under the laws of England and Wales (“Volition Diagnostics”), which was formed in November 2015, Volition America, Inc., a Delaware corporation (“Volition America”), which was formed on in February 2017, Volition Veterinary Diagnostics Development LLC, a Texas limited liability company (“Volition Vet”), which was formed in June 2019, and Volition Germany GmbH (formerly Octamer GmbH, or “Octamer” and now “Volition Germany”), a Munich, Germany-based epigenetic reagent company that it acquired in January 2020.
Our principal executive office is located at 13215 Bee Cave Parkway, Suite 125, Galleria Oaks B, Austin, Texas 78738. Our telephone number is +1 (646) 650-1351. Our website is located at www.volition.com. The information that can be accessed through our website is not incorporated by reference into this Report and should not be considered to be a part hereof.
Financial Information
See our consolidated financial statements and accompanying notes to the consolidated financial statements included in this Report.
| 12 |
| Table of Contents |
ITEM 1A. |
RISK FACTORS |
Our short and long-term success is subject to numerous risks and uncertainties, many of which involve factors that are difficult to predict or beyond our control. As a result, investing in our common stock involves substantial risk. Before deciding to purchase, hold or sell our common stock, stockholders, and potential stockholders should carefully consider the risks and uncertainties described below, in addition to the other information contained in or incorporated by reference into this Report, as well as the other information we file with the SEC. If any of these risks are realized, our business, financial condition, results of operations, and prospects could be materially and adversely affected. In that case, the value of our common stock could decline, and stockholders may lose all or part of their investment. Furthermore, additional risks and uncertainties of which we are currently unaware, or which we currently consider to be immaterial, could have a material adverse effect on our business.
Certain statements made in this section constitute “forward-looking statements,” which are subject to numerous risks and uncertainties including those described in this section. Refer to the section entitled “Cautionary Note Regarding Forward-Looking Statements” within this Report for additional information.
Risks Associated with Our Company
We operate in a rapidly changing environment that involves a number of risks that could materially affect our business, financial condition or future results, some of which are beyond our control. The summary below, as well as the discussion that follows the summary, highlights some of the risks that may affect future operating results. These are the risks and uncertainties we believe are most important for you to consider. We cannot be certain that we will successfully address these risks. If we are unable to address these risks, among other things, our business may not grow, our stock price may suffer, and we may be unable to stay in business. Additional risks and uncertainties not presently known to us, which we currently deem immaterial or which are similar to those faced by other companies in our industry or business in general, may also impair our business operations.
Risk Factor Summary
Risks Related to our Business and Business Strategy
|
· |
We have incurred significant losses, and we may never achieve profitability. |
|
· |
We may need to raise additional capital in the future. If we are unable to secure adequate funds on terms acceptable to us, we may be unable to execute our plan of operations. |
|
· |
It is difficult to forecast our future performance, which may cause our financial results to fluctuate unpredictably. |
|
· |
The cancer diagnostics market is highly competitive and subject to rapid technological change; accordingly, we will face fierce competition, including from companies with greater resources and experience than us, and our intended products may not achieve significant market penetration and/or may become obsolete. |
|
· |
Our management has broad discretion over the use of our available cash and might not allocate cash in ways that increase the value of your investment. |
|
· |
Our future success depends on our ability to retain our officers and directors, scientists, and other key employees and to attract, retain and motivate qualified personnel. |
|
· |
If any of our facilities or our laboratory equipment were damaged or destroyed, or if we experience a significant disruption in our operations for any reason, our ability to continue to operate our business could be materially harmed. |
|
· |
Failure in our information technology, storage systems or our clinical laboratory equipment could significantly disrupt our operations and our research and development efforts. |
|
· |
Our business and reputation will suffer if we are unable to establish and comply with stringent quality standards to assure that the highest level of quality is observed in the performance of our tests. |
|
· |
Declining global economic or business conditions may have a negative impact on our business. |
|
· |
The COVID-19 pandemic could adversely impact our business operations, strategy, financial performance and results of operations, the extent of which is uncertain and difficult to predict. |
|
· |
We may engage in acquisitions that are not successful and which could disrupt our business, cause dilution to our stockholders and reduce our financial resources. |
| 13 |
| Table of Contents |
Risks Related to Product Development, Commercialization and Sales of Our Products
|
· |
If the marketplace does not accept the products in our development pipeline or any other diagnostic products we might develop, we may be unable to generate sufficient revenue to sustain and grow our business. |
|
· |
Our business is dependent on our ability to successfully develop and commercialize diagnostic products. If we fail to develop and commercialize diagnostic products, we may be unable to execute our plan of operations. |
|
· |
Failure to successfully develop, manufacture, market, and sell our future products will have a material adverse effect on our business, financial condition, and results of operations. |
|
· |
The results of pre-clinical studies and completed clinical trials are not necessarily predictive of future results, and our current product candidates may not have favorable results in later studies or trials which, in turn, could have a material adverse effect on our business. |
|
· |
Our research and development efforts will be hindered if we are not able to obtain samples, contract with third parties for access to samples or complete timely enrollment in future clinical trials. |
|
· |
If the third parties on which we increasingly rely to assist us with our current and anticipated pre-clinical development or clinical trials do not perform as expected, we may not be able to obtain regulatory clearance or approval or commercialize our products. |
|
· |
We expect to expand our product development, research and sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations. |
|
· |
We have limited experience with direct sales and marketing and any failure to build and manage a direct sales and marketing team effectively, or to successfully engage third party providers for such services, could have a material adverse effect on our business. |
|
· |
We will rely on third parties to manufacture and supply our intended products. Any problems experienced by these third parties could result in a delay or interruption in the supply of our intended products to our customers, which could have a material negative effect on our business. |
|
· |
We will depend on third-party distributors to market and sell our products which will subject us to a number of risks. |
|
· |
The manufacturing operations of our third-party manufacturers will likely be dependent upon third-party suppliers, making us vulnerable to supply shortages and price fluctuations, which could harm our business. |
|
· |
Defects in our products may subject us to substantial damages which could materially harm our business or financial condition. |
Risks Related to Governmental Regulation and Reimbursement
|
· |
Our failure to obtain necessary regulatory clearances or approvals on a timely basis would significantly impair our ability to distribute and market our future products on the clinical IVD market. |
|
· |
Reductions or changes in reimbursement policies could limit our ability to sell our products. |
|
· |
If we are found to have violated laws concerning the privacy and security of patient health information or other personal information, we could be subject to civil or criminal penalties, which could increase our liabilities and harm our reputation or our business. |
Risks Related to our Intellectual Property
|
· |
If the patents we rely on to protect our intellectual property prove to be inadequate, our ability to successfully commercialize our products will be harmed and we may never be able to operate our business profitably. |
|
· |
If third parties assert that we have infringed their patents and proprietary rights or challenge the validity of our patents and proprietary rights, we may become involved in intellectual property disputes and litigation that would be costly, time consuming, and delay or prevent the development or commercialization of our products. |
|
· |
If we are unable to protect our trade secrets, we may be unable to protect our interests in proprietary technology, processes and know-how that is not patentable or for which we have elected not to seek patent protection. |
Risks Related to our Securities
|
· |
The market prices and trading volume of our stock may be volatile. |
|
· |
We have identified material weaknesses in our internal control over financial reporting that have not yet been remediated, and although we are working to address such weaknesses, the failure to address these material weaknesses, or the identification of any others, could impact the reliability of our financial reporting and harm investors’ views of us, which could adversely impact our stock price. |
|
· |
We have a “going concern” opinion from our auditors, indicating the possibility that we may not be able to continue to operate. |
| 14 |
| Table of Contents |
|
· |
Our Second Amended and Restated Certificate of Incorporation exculpates our officers and directors from certain liability to our Company and our stockholders. |
|
· |
Our corporate governance documents, and certain corporate laws applicable to us, and share ownership by executive officers and directors, could make a takeover attempt, which may be beneficial to our stockholders, more difficult. |
|
· |
We do not expect to pay dividends in the foreseeable future. |
|
· |
We may in the future issue additional shares of our common stock which would reduce investors’ ownership interests in the Company, and which may cause our stock price to decline. |
|
· |
Future sales of our common stock could depress the market price of our common stock. |
|
· |
If equity research analysts do not publish research or reports about our business, or if they do publish such reports but issue unfavorable commentary or downgrade our common stock, the price and trading volume of our common stock could decline. |
|
· |
We are a smaller reporting company and a non-accelerated filer, and we cannot be certain if the reduced disclosure requirements applicable to our filing status, as well as the exemption from the requirement to provide an auditor’s attestation report regarding the effectiveness of our internal controls, will make our common stock less attractive to investors. |
Risks Related to our Business and Business Strategy
We have incurred significant losses, and we may never achieve profitability.
We are a clinical stage company and have incurred losses since our formation. As of December 31, 2021, we have an accumulated total deficit of approximately $137 million. As we continue the discovery and development of our future diagnostic products, we expect our expenses to increase significantly. Even as we begin to market and sell our intended products, we expect our losses to continue as a result of ongoing research and development expenses, as well as increased manufacturing, sales and marketing expenses. These losses, among other things, have had and will continue to have an adverse effect on our working capital, total assets and stockholders’ equity. Because of the numerous risks and uncertainties associated with our product development and commercialization efforts, we are unable to predict when or if we will become profitable. Even if we do achieve profitability, we may not be able to sustain or increase profitability on a quarterly or annual basis. If we are unable to achieve and then maintain profitability, our business, financial condition and results of operations will be negatively affected, and the market value of our common stock will decline.
We may need to raise additional capital in the future. If we are unable to secure adequate funds on terms acceptable to us, we may be unable to execute our plan of operations.
We will require additional capital to fully fund our current strategic plan, which includes successfully commercializing our Nu.Q® cancer pipeline and developing future products. If we incur delays in commencing commercialization of our Nu.Q® cancer pipeline or other future products or in achieving significant product revenue, or if we encounter other unforeseen adverse business developments, we may exhaust our capital resources prior to the commencement of commercialization.
We cannot be certain that additional capital will be available when needed or that our actual cash requirements will not be greater than anticipated. Financing opportunities may not be available to us, or if available, may not be available on favorable terms. The availability of financing opportunities will depend on various factors, such as market conditions and our financial condition and outlook. In addition, if we raise additional funds through the issuance of equity or convertible debt securities, the percentage ownership of our stockholders could be significantly diluted, and these newly issued securities may have rights, preferences or privileges senior to those of existing stockholders. If we obtain debt financing, a substantial portion of our operating cash flow may be dedicated to the payment of principal and interest on such indebtedness, and the terms of the debt securities issued could impose significant restrictions on our operations. If we are unable to obtain financing on terms favorable to us, we may be unable to execute our plan of operations and we may be required to cease or reduce development or commercialization of any future products, sell some or all of our technology or assets or merge with another entity.
It is difficult to forecast our future performance, which may cause our financial results to fluctuate unpredictably.
Our limited operating history and the rapid evolution of the market for diagnostic products make it difficult for us to predict our future performance. A number of factors, many of which are outside of our control, may contribute to fluctuations in our financial results, such as:
|
· |
our ability to develop or procure antibodies for clinical use in our future products; |
|
· |
our ability to translate preliminary clinical results to larger prospective symptomatic and screening populations; |
|
· |
the demand for our intended products; |
|
· |
our ability to obtain any necessary financing; |
|
· |
our ability to market and sell our future products; |
| 15 |
| Table of Contents |
|
· |
market acceptance of our future products and technology; |
|
· |
performance of any future strategic business partners; |
|
· |
our ability to obtain regulatory clearances or approvals; |
|
· |
our success in collecting payments from third-party payors and customers; |
|
· |
changes in technology that may render our future products uncompetitive or obsolete; |
|
· |
competition with other cancer diagnostics companies; and |
|
· |
adverse changes in the healthcare industry (human and canine). |
The cancer diagnostics market is highly competitive and subject to rapid technological change; accordingly, we will face fierce competition, including from companies with greater resources and experience than us, and our intended products may not achieve significant market penetration and/or may become obsolete.
The cancer diagnostics market is extremely competitive and characterized by rapidly evolving industry standards and new product enhancements. Cancer diagnostic tests are technologically innovative and require significant planning, design, development, and testing at the technological, product, and manufacturing process levels. These activities require significant capital commitments and investment. There can be no assurance that our intended products or proprietary technologies will remain competitive following the introduction of new products and technologies by competing companies within the industry. Furthermore, there can be no assurance that our competitors will not develop products that render our future products obsolete or that are more effective, accurate or can be produced at lower costs. There can be no assurance that we will be successful in the face of increasing competition from new technologies or products introduced by existing companies in the industry or by new companies entering the market.
The market for cancer diagnostics is also significantly affected by new product introductions and other market activities of industry participants. Our competitors include large multinational corporations and their operating units, including Exact Sciences Corporation, Guardant Health, GRAIL Inc., Freenome Holdings Inc, CellMax Life, Archer DX Inc., Thrive Earlier Detection Corp., Foundation Medicine Inc., Oncocyte Corporation, OpKo Health Inc., MDNA Life Sciences Inc., Oncimmune Holdings Plc, Abbott Laboratories Inc., Cepheid Inc., Koninklijke Philips N.V., GE Healthcare, Siemens, Gen-Probe Incorporated, EpiGenomics AG, MDxHealth SA, and Roche Diagnostics, and from companies such as Mars Incorporated, IDEXX Laboratories Inc., PetDx, One Health Company (Fidocure) and Vidium Animal Health focused on the veterinary space. There may also be other companies developing products competitive with ours of which we are unaware.
Many of our competitors have greater resources and experience than us and may enjoy several competitive advantages, including:
|
· |
significantly greater name recognition; |
|
· |
established relationships with healthcare professionals, companies and consumers; |
|
· |
additional lines of products, and the ability to offer rebates or bundle products to offer higher discounts or incentives to gain a competitive advantage; |
|
· |
established supply and distribution networks; and |
|
· |
greater resources for product development, sales and marketing, and intellectual property protection. |
Many of these other companies have developed and will continue to develop new products that will compete directly with our future products. In addition, many of our competitors spend significantly greater funds for the research, development, promotion, and sale of new and existing products. These resources may allow them to respond more quickly to new or emerging technologies and changes in consumer requirements. We also face competition in our search for third parties to assist us with sales and marketing of our product candidates, which may negatively impact our ability to enter into favorable sales and marketing arrangements. For all the foregoing reasons, we may not be able to compete successfully against our competitors.
Our management has broad discretion over the use of our available cash and might not allocate cash in ways that increase the value of your investment.
As of December 31, 2021, we had approximately $20.6 million in combined cash and cash equivalents compared to approximately $19.4 million as of December 31, 2020. Our management expects to deploy these resources primarily to expand our commercialization activities, to fund our product development efforts and for general corporate and working capital purposes. However, our management has broad discretion to pursue other objectives. Our management might not apply our cash in ways that increase or permit any return of your investment.
Our future success depends on our ability to retain our officers and directors, scientists, and other key employees and to attract, retain and motivate qualified personnel.
Our success depends on our ability to attract, retain and motivate highly qualified management and scientific personnel. In particular, we are highly dependent on Cameron Reynolds, our President and Chief Executive Officer, our other officers and directors, scientists and key employees. The loss of any of these persons or their expertise would be difficult to replace and could have a material adverse effect on our ability to achieve our business goals. In addition, the loss of the services of any one of these persons may impede the achievement of our research, development and commercialization objectives by diverting management’s attention to the identification of suitable replacements, if any. There can be no assurance that we will be successful in hiring or retaining qualified personnel and our failure to do so could have a material adverse effect on our business, financial condition and results of operations.
| 16 |
| Table of Contents |
Recruiting and retaining qualified scientific personnel and, in the future, sales and marketing personnel will also be critical to our success. We may not be able to attract and retain these personnel on acceptable terms given the competition among pharmaceutical, biotechnology and diagnostic companies for similar personnel. We also experience competition for the hiring of scientific personnel from universities and research institutions. We do not maintain “key person” insurance on any of our employees. In addition, we rely on consultants and advisors, including scientific and clinical advisors, to assist us in formulating our research, development and commercialization strategies. Our consultants and advisors, however, may have other commitments or employment that may limit their availability to us.
If any of our facilities or our laboratory equipment were damaged or destroyed, or if we experience a significant disruption in our operations for any reason, our ability to continue to operate our business could be materially harmed.
If our present, or any future facilities, were to be damaged, destroyed or otherwise unable to operate, whether due to fire, floods, storms, tornadoes, earthquakes, other inclement weather events or natural disasters, employee malfeasance, terrorist acts, power outages, or otherwise, it may render it difficult or impossible for us to perform our research and development for some period of time and our business could be severely disrupted. The lead time from ordering to delivery of certain specialized equipment we use can be more than six months and difficult to substitute.
Failure in our information technology, storage systems or our clinical laboratory equipment could significantly disrupt our operations and our research and development efforts.
Our ability to execute our business strategy depends, in part, on the continued and uninterrupted performance of our information technology systems, which support our operations including our research and development efforts. The integrity and protection of our own data, and that of our customers, clinical trial subjects and employees, is critical to our business. The regulatory environment governing information, security and privacy laws is increasingly demanding and continues to evolve. IT systems are vulnerable to damage from a variety of sources. High-profile security breaches at other companies and in government agencies have increased in recent years, and cyber-attacks are becoming more sophisticated and frequent, and in some cases have caused significant harm. Computer hackers and others routinely attempt to breach the security of technology products, services and systems, and to fraudulently induce employees, customers, or others to disclose information or unwittingly provide access to systems or data. While we devote significant resources to security measures to protect our systems and data, these measures cannot provide absolute security.
Any breach or interruption of our information technology systems could compromise our networks and the information stored therein could be accessed by unauthorized parties, publicly disclosed, lost or stolen. Despite the precautionary measures we have taken to prevent unanticipated problems that could affect our IT systems, unauthorized access, loss or disclosure could also disrupt our operations, including our ability to:
|
· |
provide customer assistance services; |
|
· |
conduct research and development activities; |
|
· |
collect, process and prepare company financial information; |
|
· |
provide information about our tests and other patient and healthcare provider education and outreach efforts through our website; and |
|
· |
manage the administrative aspects of our business and damage to our reputation. |
Any such access, disclosure or other loss of information could result in legal claims or proceedings, liability under laws that protect the privacy of personal information, such as the U.S. Health Insurance Portability and Accountability Act of 1996, similar U.S. state data protection regulations, including the California Consumer Privacy Act, the EU’s General Data Protection Regulation, and other regulations, the breach of which could result in significant penalties.
Failure to adequately protect and maintain the integrity of our information systems and data, including as a result of a security breach, may result in significant losses and have a material adverse effect on our financial position, results of operations and cash flows.
Our business and reputation will suffer if we are unable to establish and comply with stringent quality standards to assure that the highest level of quality is observed in the performance of our tests.
Inherent risks are involved in providing and marketing diagnostic and monitoring tests and related services. Patients and healthcare providers rely on us to provide accurate clinical and diagnostic information that may be used to make critical healthcare decisions. Consequently, users of our tests may have a greater sensitivity to errors than users of some other types of products and services. We must maintain high service standards and other quality controls. Performance or accuracy defects, incomplete or improper process controls, excessively slow turnaround times, unanticipated uses of our tests or mishandling of samples or test results (whether by us, patients, healthcare providers, courier delivery services, or others) can lead to adverse outcomes for patients and interruptions to our services. These events could lead to voluntary or legally mandated safety alerts relating to our tests or our laboratory facilities and could result in the removal of our products and services from the market or the suspension of our laboratories’ operations. Insufficient quality controls and any resulting negative outcomes could result in significant costs and litigation, as well as negative publicity that could reduce demand for our tests and payers’ willingness to cover our tests. Even if we maintain adequate controls and procedures, damaging and costly errors may occur.
| 17 |
| Table of Contents |
Declining global economic conditions may have a negative impact on our business.
Concerns over U.S. healthcare reform legislation and energy costs, geopolitical issues, the availability and cost of credit and government stimulus programs in the United States and other countries may contribute to increased volatility and diminished expectations for the global economy. If the economic climate deteriorates, our business, including our access to the research use only, or clinical IVD markets for diagnostic tests, could be adversely affected, resulting in a negative impact on our business, financial condition and results of operations.
The United Kingdom’s withdrawal from the European Union became effective in January 2021. Although it is known what the terms of this withdrawal were, it is still possible that greater restrictions on imports and exports between the European Union countries and the United Kingdom and increased regulatory complexities are forthcoming. These changes may adversely affect our ability to market our future products in the United Kingdom which could have an adverse effect on our business, financial condition, and results of operations.
In addition, following Russia’s military invasion of Ukraine in February 2022, NATO deployed additional military forces to Eastern Europe, and the United States, European Union, and other nations announced various sanctions against Russia. The invasion of Ukraine and the retaliatory measures that have been taken, and could be taken in future, by the U.S., NATO, and other countries have created global security concerns that could result in a regional conflict and otherwise have a lasting impact on regional and global economies, any or all of which could adversely affect our business.
The COVID-19 pandemic could adversely impact our business operations, strategy, financial performance and results of operations, the extent of which is uncertain and difficult to predict.
As a result of the COVID-19 pandemic and the related responses from government authorities, we have experienced and may continue to experience disruptions that could severely impact our business, strategy, financial performance and financial condition, as well as clinical trials, including:
|
· |
delays or difficulties in enrolling patients in clinical trials; |
|
· |
delays or difficulties in clinical site initiation, including difficulties in recruiting clinical site investigators and clinical site staff; |
|
· |
diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as clinical trial sites and hospital staff supporting the conduct of our clinical trials; |
|
· |
interruption of key clinical trial activities, such as clinical trial site monitoring, due to limitations on travel imposed or recommended by governments, employers and others; |
|
· |
limitations in employee resources, including because of sickness of employees or their families or the desire of employees to avoid contact with large groups of people; |
|
· |
disruptions to our operations, including a shutdown of one or more of our facilities; restrictions on our operations and sales, marketing and distribution efforts; and interruptions to our research and development, manufacturing, clinical/regulatory and other important business activities; |
|
· |
increased costs in our manufacturing, production and shipping processes; |
|
· |
a slowdown or stoppage in the supply chain of the raw materials, components, and packaging services used to manufacture our products or our inability to secure additional or alternate sources of supplies or services needed to manufacture our products at optimal levels; |
|
· |
interruptions or delays in global shipping to transport and deliver our products to our distributors and customers; |
|
· |
interruptions in normal operations of certain end user customers that could result in reductions in demand for routine, elective and other non-COVID-19 related healthcare procedures and testing; |
|
· |
limitations on employee resources and availability, including due to sickness or personal quarantine, government restrictions, the desire of employees to avoid contact with large groups of people, or school closures or remote learning; |
|
· |
a COVID-19 vaccination mandate or requirement that unvaccinated employees be tested frequently could result in employee attrition and difficulty securing future labor needs, including attrition of critically skilled labor, difficulty in obtaining services from impacted suppliers and increased costs; and |
|
· |
fluctuations in foreign currency exchange rates or interest rates resulting from market uncertainties. |
| 18 |
| Table of Contents |
The COVID-19 pandemic has resulted in government authorities implementing many measures to contain the spread of COVID-19, including travel bans and restrictions, quarantines, shelter-in-place and stay-at-home orders, and business and school shutdowns. Although many of these measures have been lifted or relaxed, they could be reinstituted if conditions deteriorate and could be in place for a significant period of time, which could adversely affect our operations. For example, at the outset of the pandemic, we temporarily closed our corporate offices and had personnel work remotely to the extent possible and may be required to do so again in the future. Further, our sales and marketing activities were, and may continue to be, adversely affected by the inability to conduct in-person sales activities, meetings, events and conferences, which could negatively impact the success of our sales and marketing strategies and our relationships with our customers.
The continued spread of COVID-19 has also led to disruption and volatility in the global capital markets, which increases the cost of, and adversely impacts access to, capital and increases economic uncertainty. This volatility and uncertainty may adversely affect our stock price. The actions that governments and individuals have taken in response to COVID-19 have led to a sharp contraction in many aspects of economies worldwide. The pandemic may cause an economic slowdown of potentially extended duration, and it is possible that it could cause a global recession. If this occurs, it could negatively impact our ability to develop and commercialize our products, among other things. Even after the COVID-19 pandemic has subsided, we may continue to experience material adverse effects to our business as a result of the global economic impact of the pandemic.
The effects of COVID-19 may exacerbate our other risk factors described in this Report. The degree to which the COVID-19 pandemic may impact our business and clinical trials and development activities will depend on future developments, which are highly uncertain, continuously evolving and cannot be predicted with confidence, such as the ultimate duration of the pandemic, the severity of continual outbreak surges and variants, travel restrictions and social distancing requirements in the countries where we conduct business, the effectiveness of actions taken to contain and treat the disease, and how quickly and to what extent more normalized economic and operating conditions can resume. Because this situation continues to evolve globally, the ultimate impacts to us of COVID-19 are uncertain, but such impacts could have a material adverse effect on our business, strategy, financial performance and financial condition.
We may engage in acquisitions that are not successful and which could disrupt our business, cause dilution to our stockholders and reduce our financial resources.
From time to time, we may consider opportunities to acquire or invest in other companies, products or technologies that may enhance our product platform or technology, expand the breadth of our markets or customer base, or otherwise advance our business strategies. Potential and completed acquisitions and investments involve numerous risks, including the following:
|
· |
we may be unable to successfully integrate the acquired business (es) into our business; |
|
· |
we may be unable to realize the anticipated benefits of the acquisition; |
|
· |
the acquisition may not strengthen our competitive position; and |
|
· |
our future results may suffer if we do not effectively manage our expanded operations. |
We do not know if we will be able to identify future acquisitions or investments we deem suitable, whether we will be able to successfully complete any such acquisitions or investments on favorable terms or at all, or whether we will be able to successfully integrate any acquired products or technologies into our business. Our potential inability to integrate any acquired products or technologies effectively may adversely affect our business, operating results and financial condition.
Risks Related to Product Development, Commercialization and Sales of Our Products
If the marketplace does not accept the products in our development pipeline or any other diagnostic products we might develop, we may be unable to generate sufficient revenue to sustain and grow our business.
Our intended products may never gain significant acceptance in the research or clinical marketplace and therefore may never generate substantial revenue or profits. Physicians, hospitals, clinical laboratories, researchers or others in the healthcare industry may not use our future products unless they determine that they are an effective and cost-efficient means of detecting and diagnosing cancer. If our research and studies do not satisfy providers, payors and others as to the reliability and effectiveness, we may experience reluctance or refusal on the part of the physician to use our future products. In addition, we will need to expend a significant amount of resources on marketing and educational efforts to create awareness of our future products and to encourage their acceptance and adoption. If the market for our future products does not develop sufficiently or the products are not accepted, our revenue potential will be harmed.
| 19 |
| Table of Contents |
Our business is dependent on our ability to successfully develop and commercialize diagnostic products. If we fail to develop and commercialize diagnostic products, we may be unable to execute our plan of operations.
Our current business strategy focuses on discovering, developing and commercializing diagnostic products. The success of our business will depend on our ability to fully develop and commercialize the diagnostic products in our current development pipeline as well as continue the discovery and development of other diagnostics products.
Prior to commercializing the Nu.Q® tests and other diagnostic products, we will be required to undertake time-consuming and costly development activities with uncertain outcomes, including conducting clinical studies and obtaining regulatory clearance or approval in the United States, Asia and in Europe. Delays in obtaining approvals and clearances could have material adverse effects on us and our ability to fully carry out our plan of operations. We have limited experience in taking products through these processes and there are considerable risks involved in these activities. The science and methods that we are employing are innovative and complex, and it is possible that our development programs will ultimately not yield products suitable for commercialization or government approval. Products that appear promising in early development may fail to be validated in subsequent studies, and even if we achieve positive results, we may still fail to obtain the necessary regulatory clearances or approvals. Few research and development projects result in commercial products, and perceived viability in early clinical studies often is not replicated in later studies. At any point, we may abandon development of a product, or we may be required to expend considerable resources obtaining additional clinical and nonclinical data, which would adversely impact the timing for generating potential revenue from those products. Further, our ability to develop and launch diagnostic tests is dependent on our receipt of substantial additional funding. If our discovery and development programs yield fewer commercial products than we expect, we may be unable to execute our business plan, and our business, financial condition and results of operations may be adversely affected.
Failure to successfully develop, manufacture, market, and sell our future products will have a material adverse effect on our business, financial condition, and results of operations.
We are in the process of developing a suite of diagnostic tests as well as additional products. The successful development and commercialization of our intended products is critical to our future success. Our ability to successfully develop, manufacture, market, and sell our future products is subject to a number of risks, many of which are outside our control. There can be no assurance that we will be able to develop and manufacture products in commercial quantities at acceptable costs, successfully market any products, or generate revenues from the sale of any products. Failure to achieve any of the foregoing would have a material adverse effect on our business, financial condition, and results of operations.
The results of pre-clinical studies and completed clinical trials are not necessarily predictive of future results, and our current product candidates may not have favorable results in later studies or trials which, in turn, could have a material adverse effect on our business.
We must conduct extensive testing of our product candidates and new indications of our marketed products before we can obtain regulatory approval to market and sell them. Success in pre-clinical studies or completed clinical trials does not ensure that later studies or trials, including continuing pre-clinical studies and large-scale clinical trials, will be successful nor does it necessarily predict future results. Favorable results in early studies or trials may not be repeated in later studies or trials, and product candidates in later stage trials may fail to show acceptable safety and efficacy despite having progressed through earlier trials. We may be required to demonstrate through large, long-term outcome trials that our product candidates are safe and effective for use in a broad population prior to obtaining regulatory approval. The failure of clinical trials to demonstrate the safety and effectiveness of our clinical candidates for the desired indication(s) would preclude the successful development of those candidates for such indication(s), in which event our business, prospects, results of operations and financial condition may be adversely affected.
Our research and development efforts will be hindered if we are not able to obtain samples, contract with third parties for access to samples or complete timely enrollment in future clinical trials.
Access to human and animal sample types, such as blood is necessary for our research and product development. Acquiring samples from individuals / animals with clinical diagnoses or associated clinical outcomes through purchase or clinical studies is necessary. Lack of available samples can delay development timelines and increase costs of development. Generally, the agreements under which we gain access to human and animal samples are non-exclusive. Other companies may compete with us for access. If we are not able to negotiate access to clinical samples with research institutions, hospitals, clinical partners, pharmaceutical companies, or companies developing therapeutics and/or diagnostics on a timely basis, or at all, or if other laboratories or our competitors secure access to these samples before us, our ability to research, develop and commercialize future products will be limited or delayed. Equally, we may not be able to conduct or complete clinical studies in a timely manner if we are unable to enroll sufficient numbers of patients in such studies, which could consequently have an adverse effect on our research and development and product commercialization efforts.
| 20 |
| Table of Contents |
If the third parties on which we increasingly rely to assist us with our current and anticipated pre-clinical development or clinical trials do not perform as expected, we may not be able to obtain regulatory clearance or approval or commercialize our products.
As our clinical infrastructure expands, we expect to increasingly rely on third parties, such as contract research organizations, medical institutions, clinical investigators and contract laboratories to conduct some of our current and anticipated pre-clinical investigations and clinical trials. If we are not able to reach mutually acceptable agreements with these third parties on a timely basis, these third parties do not successfully carry out their commitments or regulatory obligations or meet expected deadlines, or the quality or accuracy of the data they obtain is compromised due to the failure to adhere to agreed-upon clinical protocols or regulatory requirements or for other reasons, our pre-clinical development activities or clinical trials may be extended, delayed, suspended or terminated, and we may not be able to obtain regulatory clearance or approval for, or successfully commercialize, our products on a timely basis, if at all, and our business, operating results and prospects may be adversely affected.
We expect to expand our product development, research and sales and marketing capabilities, and as a result, we may encounter difficulties in managing our growth, which could disrupt our operations.
We are focused on developing our pipeline for future products. It is likely that our efforts will result in significant growth in the number of our consultants, advisors, and employees, in addition to the scope of our operations. In order to manage our anticipated future growth, we must continue to implement and improve our managerial, operational and financial systems, expand our facilities, and continue to recruit and train additional qualified personnel. Due to our limited resources, we may not be able to effectively manage the expansion of our operations or recruit and train additional qualified personnel. The expansion of our operations may lead to significant costs and may divert our management and business development resources. Any inability to manage growth could delay the execution of our business plan or disrupt our operations.
We have limited experience with direct sales and marketing and any failure to build and manage a direct sales and marketing team effectively, or to successfully engage third party providers for such services, could have a material adverse effect on our business.
As an organization we have limited experience with direct sales however are building a team of experienced individuals in terms of market intelligence, product management and account management in addition to building connections with market-leading established distributors as potential commercial partners. Our products will require several dynamic and evolving sales models tailored to different worldwide markets, users and products. Our sales strategy is initially focused on the clinical IVD market with the CE marking of our first product in Europe. Following CE marking of our first product in Europe we intend to enter the European markets and, following the completion of any necessary regulatory clearances, certain Asian markets. Even if we receive a CE mark, we must still seek regulatory clearance in other jurisdictions. A failure to obtain these regulatory clearances in other jurisdictions could negatively affect our business. Pending completion of our review of the regulatory environment in the United States we may decide to enter the United States market through a Clinical Laboratory Improvement Amendments (“CLIA”), certified laboratory located in the United States. We remain firmly committed to pursuing FDA approval as our primary objective. FDA approval can consist of PMA or 510(k) clearance depending on the test complexity and risk posed to patients. We intend to pursue the most appropriate approval pathway for each individual product developed. We intend to progressively grow to large volumes of tests sold to centralized laboratories and eventually reach the mass diagnostics testing market. The exact nature of the ideal sales strategy will evolve as we continue to develop our intended products and seek entry into the IVD markets. We also have limited experience with direct sales and marketing and we intend to engage a network of distributors to help commercialize our products worldwide. Any failure to build and manage a direct sales and marketing team effectively, or to successfully engage third-party providers for such services, could have a material adverse effect on our business.
There are significant risks involved in building and managing our sales and marketing organization, as well as identifying and negotiating deals with the right sales and distribution partners, including risks related to our ability to:
|
· |
identify appropriate partners; |
|
· |
negotiate beneficial partnership and distribution agreements; |
|
· |
hire qualified individuals as needed; |
|
· |
generate sufficient leads within our targeted market for our sales force; |
|
· |
provide adequate training for effective sales and marketing; |
|
· |
protect intellectual property rights; |
|
· |
retain and motivate our direct sales and marketing professionals; and |
|
· |
effectively oversee geographically dispersed sales and marketing teams. |
Our failure to adequately address these risks could have a material adverse effect on our ability to increase sales and use of our future products, which would cause our revenues to be lower than expected and harm our results of operations. Further, we are required to comply with numerous other federal, state, and local laws relating to matters such as safe working conditions, industrial safety, and labor laws. We may incur significant costs to comply with such laws and regulations in the future, and lack of compliance could have material adverse effects on our operations. We believe that we have structured our business operations to comply with applicable legal requirements. However, it is possible that governmental entities or other third parties could interpret these laws differently and assert otherwise, which could have a material adverse impact on our business.
| 21 |
| Table of Contents |
We will rely on third parties to manufacture and supply our intended products. Any problems experienced by these third parties could result in a delay or interruption in the supply of our intended products to our customers, which could have a material negative effect on our business.
We will rely on third parties to manufacture and supply our intended products. The manufacture of our intended diagnostic products will require specialized equipment and utilize complicated production processes that would be difficult, time-consuming and costly to duplicate. If the operations of third-party manufacturers are interrupted or if they are unable to meet our delivery requirements due to capacity limitations or other constraints, we may be limited in our ability to fulfill our future sales orders. Any prolonged disruption in the operations of third-party manufacturers could have a significant negative impact on our ability to sell our future products, could harm our reputation and could cause us to seek other third-party manufacturing contracts, thereby increasing our anticipated development and commercialization costs. In addition, if we are required to change manufacturers for any reason, we will be required to verify that the new manufacturer maintains facilities and procedures that comply with quality standards required by the FDA and with all applicable regulations and guidelines. The delays associated with the verification of a new manufacturer could negatively affect our ability to develop products or receive approval of any products in a timely manner.
We will depend on third-party distributors to market and sell our products, which will subject us to a number of risks.
We will depend on third-party distributors to market, sell, and service our products in our intended markets. We are subject to a number of risks associated with reliance upon third-party distributors including the following:
|
· |
lack of day-to-day control over the activities of third-party distributors; |
|
· |
third-party distributors may not commit the necessary resources to market and sell our products to our level of expectations; |
|
· |
third-party distributors may terminate their arrangements with us on limited or no notice or may change the terms of these arrangements in a manner unfavorable to us; and |
|
· |
disagreements with our distributors could result in costly and time-consuming litigation or arbitration which we could be required to conduct in jurisdictions with which we are not familiar. |
If we fail to establish and maintain satisfactory relationships with our third-party distributors, our revenues and market share may not grow as anticipated, and we could be subject to unexpected costs which could harm our results of operations and financial condition.
The manufacturing operations of our third-party manufacturers will likely be dependent upon third-party suppliers, making us vulnerable to supply shortages and price fluctuations, which could harm our business.
The operations of our future third-party manufacturers will likely be dependent upon third-party suppliers. A supply interruption or an increase in demand beyond a supplier’s capabilities could harm the ability of our future manufacturers to manufacture our intended products until new sources of supply are identified and qualified.
Reliance on these suppliers could subject us to a number of risks that could harm our business, including:
|
· |
interruption of supply resulting from modifications to or discontinuation of a supplier’s operations; |
|
· |
delays in product shipments resulting from uncorrected defects, reliability issues, or a supplier’s variation in a component; |
|
· |
a lack of long-term supply arrangements for key components with our suppliers; |
|
· |
inability to obtain adequate supply in a timely manner, or to obtain adequate supply on commercially reasonable terms; |
|
· |
difficulty and cost associated with locating and qualifying alternative suppliers for components in a timely manner; |
|
· |
production delays related to the evaluation and testing of products from alternative suppliers, and corresponding regulatory qualifications; |
|
· |
delay in delivery due to suppliers prioritizing other customer orders over ours; |
|
· |
damage to our brand reputation caused by defective components produced by the suppliers; and |
|
· |
fluctuation in delivery by the suppliers due to changes in demand from us or their other customers. |
We have implemented certain risk mitigation strategies including the diversification of suppliers by region and the internalization of certain production processes. However, any interruption in the supply of components of our future products or materials, or our inability to obtain substitute components or materials from alternate sources at acceptable prices in a timely manner, could impair our ability to meet the demand of our future customers, which would have an adverse effect on our business.
Defects in our products may subject us to substantial damages which could materially harm our business or financial condition.
The products we develop could lead to product liability claims based on allegations that one or more of our products contained a design or manufacturing defect which resulted in the failure to detect the disease for which it was designed. A product liability claim could result in substantial damages and be costly and time consuming to defend, either of which could materially harm our business or financial condition. We cannot assure you that our product liability insurance would protect our assets from the financial impact of defending a product liability claim. Any product liability claim brought against us, with or without merit, could increase our product liability insurance rates or prevent us from securing insurance coverage in the future.
| 22 |
| Table of Contents |
Risks Related to Governmental Regulation and Reimbursement
Our failure to obtain necessary regulatory clearances or approvals on a timely basis would significantly impair our ability to distribute and market our future products on the clinical IVD market.
We are subject to regulation by the FDA in the United States, the CE in Europe, the CFDA in China, and other regulatory bodies in other countries where we intend to sell our future products. Before we are able to place our intended products in the clinical IVD markets in the United States, China and Europe, we will be required to obtain clearance or approval of our future products from the FDA and the CFDA with respect to the United States and China, respectively, and receive a CE mark with respect to Europe. In 2017, the European Union adopted the phased-in EU IVDR that may impose additional requirements to obtain a CE mark, which could result in delays and further expense, in terms of staff costs to us as compared to the current CE mark process. The EU IVDR will require each product submission to be thoroughly audited by Notified Bodies, instead of the current self-certification process. The EU IVDR will be fully applicable in May 2022.
Additionally, even if we receive the required government clearance or approval of our intended products, we are still subject to continuing regulation and oversight. Under the FDA, diagnostics are considered medical devices and are subject to ongoing controls and regulations, including inspections, compliance with established manufacturing practices, device-tracking, record-keeping, advertising, labeling, packaging, and compliance with other standards. The process of complying with such regulations with respect to current and new products can be costly and time-consuming. Failure to comply with these regulations could jeopardize our ability to sell our products and result in enforcement actions such as fines, civil penalties, injunctions, warning letters, recalls of products, delays in the introduction of products into the market, refusal of the FDA or other regulators to grant future clearances or approvals, delays by the FDA or other regulators in granting clearances or approvals, and the suspension or withdrawal of existing approvals by the FDA or other regulators, any of which could have a material adverse effect on our business, financial condition, and results of operations. Furthermore, any FDA regulations governing our future products are subject to change at any time, which may cause delays and have material adverse effects on our operations. In Europe, IVD companies are currently able to self-certify that they meet the appropriate regulatory requirements (which are subject to change with the EU MDR and the EU IVDR noted above) but are subject to inspection for enforcement. European national agencies, such as customs authorities and/or the Departments of Health, Industry and Labor, conduct market surveillance to ensure the applicable requirements have been met for products marketed within the European Union.
Reductions or changes in reimbursement policies could limit our ability to sell our products.
Market acceptance and sales of our products will depend, in part, on reimbursement policies and may be affected by healthcare reform measures. Government authorities and third-party payers, such as private health insurers and health maintenance organizations, decide which products they will pay for and establish reimbursement levels for those products. To manage healthcare costs, many governments and third-party payers in the United States increasingly scrutinize the pricing of new products and require greater levels of evidence of favorable clinical outcomes and cost-effectiveness before extending coverage. We cannot be sure that reimbursement will be available for our products and, if reimbursement is available, the level of such reimbursement. Reimbursement may impact the demand for, or the price of, our products. If reimbursement is not available or is available only at limited levels, we may not be able to successfully commercialize our future products.
If we are found to have violated laws concerning the privacy and security of patient health information or other personal information, we could be subject to civil or criminal penalties, which could increase our liabilities and harm our reputation or our business.
There are a number of U.S. and international laws protecting the privacy and security of personal information. These laws include the U.S. Health Insurance Portability and Accountability Act of 1996 (“HIPAA”) and related regulations, U.S. state laws (such as the California Consumer Privacy Act (“CCPA”)), Canada’s Personal Information Protection and Electronic Documents Act (“PIPEDA”) or the applicable provincial alternatives, the EU’s General Data Protection Regulation (“GDPR”), EU member states directives, or similar applicable laws. These laws place limits on how we may collect, use, share and store medical information and other personal information, and they impose obligations to protect that information against unauthorized access, use, loss, and disclosure.
If we, or any of our service providers who have access to the personal data for which we are responsible, are found to be in violation of the privacy or security requirements of HIPAA, PIPEDA, GDPR, or applicable foreign, U.S. state and Canadian provincial laws, we could be subject to civil or criminal penalties, which could increase our liabilities, harm our reputation and have a material adverse effect on our business, financial condition and operating results. In addition, entities operating in the healthcare industry have increasingly become targets for hackers. Although we utilize a variety of measures to secure the data that we control, even compliant entities can experience security breaches or have inadvertent failures despite employing reasonable practices and safeguards.
| 23 |
| Table of Contents |
We may also face new risks relating to data privacy and security as the United States, individual U.S. states or Canadian provinces, E.U. member states, and other international jurisdictions adopt or implement new data privacy and security laws and regulations as we continue to commercialize our products worldwide. For example, amendments to privacy and security laws (such as the CCPA) may impose additional requirements on us and increase our regulatory and litigation risk. As we continue to expand, our business will need to adapt to meet these and other similar legal requirements.
Risks Related to our Intellectual Property
If the patents we rely on to protect our intellectual property prove to be inadequate, our ability to successfully commercialize our products will be harmed and we may never be able to operate our business profitably.
Our success depends, in large part, on our ability to protect proprietary methods, discoveries and technologies that we develop under the patents and intellectual property laws of the United States, Europe and other countries, so that we can seek to prevent others from unlawfully using our inventions and proprietary information. Our patent portfolio includes 29 patent families and a total 84 patents granted related to our diagnostic tests (including veterinary applications), with 12 patents granted in the United States, 14 patents granted in Europe and a further 58 patents granted worldwide. Additionally, we have 93 patent applications pending worldwide.
If we are not able to protect our proprietary technology and information, our competitors may use our inventions to develop competing products. We cannot assure you that any of the pending patent applications will result in patents being issued. In addition, due to technological changes that may affect our products or judicial interpretation of the scope of our patents, our products might not, now or in the future, be adequately covered by our patents.
If third parties assert that we have infringed their patents and proprietary rights or challenge the validity of our patents and proprietary rights, we may become involved in intellectual property disputes and litigation that would be costly, time consuming, and delay or prevent the development or commercialization of our products.
Our ability to commercialize our products depends on our ability to develop, manufacture, market and sell our products without infringing the proprietary rights of third parties. Third parties may allege that our products or our methods or discoveries infringe their intellectual property rights. Numerous United States and foreign patents and pending patent applications, which are owned by third parties, exist in fields that relate to our products and our underlying methodologies, discoveries and technologies. A third party may sue us for infringing its patent rights.
Our ability to successfully commercialize our products depends on our ability to protect our proprietary technology and information. Likewise, we may need to resort to litigation to enforce a patent issued or licensed to us or to determine the scope and validity of third-party proprietary rights. In addition, a third party may claim that we have improperly obtained or used its confidential or proprietary information. The cost to us of any litigation or other proceeding relating to intellectual property rights, even if resolved in our favor, could be substantial, and the litigation could divert our management’s attention from other aspects of our business. Some of our competitors may be able to sustain the costs of complex patent litigation more effectively than we can because they have substantially greater resources. Uncertainties resulting from the initiation and continuation of any litigation could limit our ability to continue our operations. Additionally, we cannot be certain of the level of protection, if any that will be provided by our patents if they are challenged in court, where our competitors may raise defenses such as invalidity, unenforceability or possession of a valid license.
If we are found to infringe upon intellectual property rights of third parties, we might be forced to pay damages, potentially including triple damages. In addition to any damages, we might have to pay, a court could require us to stop the infringing activity or obtain a license. Any license required under any patent may not be made available on commercially acceptable terms, if at all. In addition, such licenses are likely to be non-exclusive and, therefore, our competitors may have access to the same technology licensed to us. If we fail to obtain a required license and are unable to design around a patent, we may be unable to effectively market some or all of our products, which could limit our ability to generate revenue or achieve profitability and possibly prevent us from generating revenue sufficient to sustain our operations.
If we are unable to protect our trade secrets, we may be unable to protect our interests in proprietary technology, processes and know-how that is not patentable or for which we have elected not to seek patent protection.
In addition to patented technology, we rely upon trade secret protection to protect our interests in proprietary know-how and for processes for which patents are difficult or impossible to obtain or enforce. We may not be able to protect our trade secrets adequately. Although we use reasonable efforts to protect our trade secrets, our employees, consultants, contractors and outside scientific advisors may unintentionally or willfully disclose our information to competitors. Enforcing a claim that a third party illegally obtained and is using any of our trade secrets is expensive and time consuming, and the outcome is unpredictable. In addition, courts outside the United States are sometimes less willing to protect trade secrets. We rely, in part, on non-disclosure and confidentiality agreements with our employees, consultants and other parties to protect our trade secrets and other proprietary technology. These agreements may be breached, and we may not have adequate remedies for any breach. Moreover, others may independently develop equivalent proprietary information, and third parties may otherwise gain access to our trade secrets and proprietary knowledge. Any disclosure of confidential information into the public domain or to third parties could allow our competitors to learn our trade secrets and use the information in competition against us, which could adversely affect our competitive advantage.
| 24 |
| Table of Contents |
Risks Related to our Securities
The market prices and trading volume of our stock may be volatile.
The market price of our common stock is likely to be highly volatile and the trading volume may fluctuate and cause significant price variation to occur. We cannot assure you that the market prices of our common stock will not fluctuate or decline significantly in the future. Some of the factors that could negatively affect the prices of our shares or result in fluctuations in those prices or in trading volume of our common stock could include the following, many of which will be beyond our control:
|
· |
competition; |
|
· |
comments by securities analysts regarding our business or prospects; |
|
· |
additions or departures of key personnel; |
|
· |
our ability to execute our business plan; |
|
· |
issuance of common stock or other securities; |
|
· |
operating results that fall below expectations; |
|
· |
loss of any strategic relationship; |
|
· |
industry developments; |
|
· |
economic and other external factors; and |
|
· |
period-to-period fluctuations in our financial results. |
In addition, the securities markets have from time-to-time experienced significant price and volume fluctuations that are unrelated to the operating performance of particular companies. These market fluctuations may also materially and adversely affect the market price and trading volume of our common stock.
We have identified material weaknesses in our internal control over financial reporting that have not yet been remediated, and although we are working to address such weaknesses, the failure to address these material weaknesses, or the identification of any others, could impact the reliability of our financial reporting and harm investors’ views of us, which could adversely impact our stock price.
Our management is responsible for establishing and maintaining adequate internal control over financial reporting. As defined in Exchange Act Rule 13a-15(f), internal control over financial reporting is a process designed by, or under the supervision of, the principal executive and principal financial officer and effected by the board of directors, management and other personnel, to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with generally accepted accounting principles and includes those policies and procedures that:
|
· |
pertain to the maintenance of records that in reasonable detail accurately and fairly reflect our transactions and dispositions of assets; |
|
· |
provide reasonable assurance that transactions are recorded as necessary to permit preparation of financial statements in accordance with generally accepted accounting principles, and that our receipts and expenditures are being made only in accordance with authorizations of our management and/or directors; and |
|
· |
provide reasonable assurance regarding prevention or timely detection of unauthorized acquisition, use or disposition of our assets that could have a material effect on the financial statements. |
We have determined that we have material weaknesses in our internal control over financial reporting as of December 31, 2021. See Part II, Item 9A of this Report for a complete discussion of these material weaknesses in our internal control over financial reporting and remediation efforts. Although we have taken and continue to take steps to address these material weaknesses, the existence of a material weakness is an indication that there is more than a remote likelihood that a material misstatement of our financial statements will not be prevented or detected in the current or any future period. There can be no assurance that we will be able to fully implement our plans and controls, as further described in Item 9A, to address these material weaknesses, or that the plans and controls, if implemented, will be successful in fully remediating these material weaknesses. In addition, we may in the future identify further material weaknesses in our internal control over financial reporting that we have not discovered to date. If we fail to successfully remediate the identified material weaknesses, or we identify further material weaknesses in our internal controls, the market’s confidence in our financial statements could decline and the market price of our common stock could be adversely impacted. Additionally, for so long as we remain as a smaller reporting company, under current rules our accounting firm will not be required to provide an opinion regarding our internal controls over financial reporting.
| 25 |
| Table of Contents |
We have a “going concern” opinion from our auditors, indicating the possibility that we may not be able to continue to operate.
Our independent registered public accountants have expressed substantial doubt about our ability to continue as a going concern. This opinion could materially limit our ability to raise additional funds by issuing new debt or equity securities or otherwise. If we fail to raise sufficient capital when needed, we will not be able to complete our proposed business plan. As a result, we may have to liquidate our business and investors may lose their investments. Our ability to continue as a going concern is dependent upon our ability to successfully accomplish our plan of operations described herein, obtain financing and eventually attain profitable operations. Investors should consider our independent registered public accountant’s comments when deciding whether to invest in the Company.
Our Second Amended and Restated Certificate of Incorporation exculpates our officers and directors from certain liability to our Company and our stockholders.
Our Second Amended and Restated Certificate of Incorporation contains a provision limiting the liability of our officers and directors for their acts or failures to act, except for acts involving intentional misconduct, fraud or a knowing violation of law. This limitation on liability may reduce the likelihood of derivative litigation against our officers and directors and may discourage or deter our stockholders from suing our officers and directors based upon breaches of their duties to our Company.
Our corporate governance documents, certain corporate laws applicable to us, and share ownership by executive officers and directors, could make a takeover attempt, which may be beneficial to our stockholders, more difficult.
Our board of directors has the power, under our charter documents to:
|
· |
issue additional shares of common stock without having to obtain stockholder approval for such action; |
|
· |
fill vacant directorships except for vacancies created by the removal of a director; |
|
· |
amend our bylaws without stockholder approval subject to certain exceptions; and |
|
· |
require compliance with an advance notice procedure with regard to business to be brought by a stockholder before an annual or special meeting of stockholders and with regard to the nomination by stockholders of candidates for election as directors. |
Further, our executive officers and directors beneficially own an amount of our outstanding shares of common stock such that if they were collectively to oppose a third party’s acquisition proposal for, or a change in control of, the Company, such officers and directors may have sufficient voting power to be able to block or at least delay such an acquisition or change in control from taking place, even if other stockholders would support such a sale or change of control.
These provisions and circumstances may discourage potential acquisition proposals and could delay or prevent a change of control, including under circumstances in which our stockholders might otherwise receive a premium over the market price of our common stock.
We do not expect to pay dividends in the foreseeable future.
We have never declared or paid cash dividends on our common stock. We do not intend to declare dividends for the foreseeable future, as we anticipate that we will reinvest any future earnings in the development and growth of our business. Therefore, investors will not receive any funds unless they sell their common stock, and stockholders may be unable to sell their shares on favorable terms or at all. We cannot assure you of a positive return on investment or that you will not lose the entire amount of your investment in our common stock.
We may in the future issue additional shares of our common stock which would reduce investors’ ownership interests in the Company, and which may cause our stock price to decline.
Our Second Amended and Restated Certificate of Incorporation authorizes the issuance of 100,000,000 shares of common stock, par value $0.001 per share. The future issuance of all or part of our remaining authorized common stock may result in substantial dilution in the percentage of our common stock held by our then existing stockholders. We may value any common stock issued in the future on an arbitrary basis. The issuance of common stock for future services or acquisitions or other corporate actions may have the effect of diluting the percentage ownership of our stockholders and, depending upon the prices at which such shares are sold or issued, on their investment in our common stock and, therefore, could have an adverse effect on any trading market for our common stock.
Future sales of our common stock could depress the market price of our common stock.
Sales of a substantial number of shares of our common stock in the public market or the perception that large sales of our shares could occur, could cause the market price of our common stock to decline or limit our future ability to raise capital through an offering of equity securities.
| 26 |
| Table of Contents |
If equity research analysts do not publish research or reports about our business, or if they do publish such reports but issue unfavorable commentary or downgrade our common stock, the price and trading volume of our common stock could decline.
The trading market for our common stock could be affected by whether and to what extent equity research analysts publish research or reports about us and our business. If one or more equity analysts cover us and publish research reports about our common stock, the price of our stock could decline rapidly if one or more securities analysts downgrade our stock or if those analysts’ issue or offer unfavorable commentary or cease publishing reports about us. If any of these analysts ceases coverage of us, we could lose visibility in the market, which in turn could cause our common stock price or trading volume to decline and our common stock to be less liquid.
We are a smaller reporting company and a non-accelerated filer and we cannot be certain if the reduced disclosure requirements applicable to our filing status, as well as the exemption from the requirement to provide an auditor’s attestation report regarding the effectiveness of our internal controls, will make our common stock less attractive to investors.
We are a “smaller reporting company,” meaning that we are not an investment company, an asset-backed issuer, or a majority-owned subsidiary of a parent company that is not a smaller reporting company and have a public float of less than $250 million measured as of the last business day of our most recently completed second fiscal quarter. “Smaller reporting companies” are able to provide simplified executive compensation disclosures in their filings and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in annual reports. We are also a “non-accelerated filer,” meaning that although we have a public float of more than $75 million measured as of the last business day of our most recently completed second fiscal quarter, our annual revenues are less than $100 million. As a “non-accelerated filer,” we are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act requiring that independent registered public accounting firms provide an attestation report on the effectiveness of internal control over financial reporting. Decreased disclosures in our SEC filings due to our status as a “smaller reporting company” and as a “non-accelerated filer” may make it harder for investors to analyze our results of operations and financial prospects and may make our common stock a less attractive investment.
| 27 |
| Table of Contents |
ITEM 1B. |
UNRESOLVED STAFF COMMENTS |
None.
ITEM 2. |
PROPERTIES |
Listed below are our current facilities as of December 31, 2021:
Location |
|
Primary Function |
|
Approx. Square Feet |
|
Leased or Owned |
Namur, Belgium (1) |
|
Research and development |
|
17,300 |
|
Owned |
Namur, Belgium (2) |
|
Manufacturing |
|
9,688 |
|
Owned |
London, UK (3) |
|
Sales and marketing |
|
323 |
|
Leased, expiring 2024 |
Triple One, Singapore (4) |
|
Sales and executive |
|
420 |
|
Leased, expiring 2022 |
Austin, Texas (5) |
|
Executive suite |
|
1,238 |
|
Leased, expiring 2022 |
(1) |
Belgian Volition purchased property located in Namur, Belgium, in October 2016, to be used as a laboratory facility for R&D. The purchase price for the property was €1.2 million, exclusive of any closing costs. |
|
|
(2) |
Belgian Volition purchased property located in Namur, Belgium, in December 2020, to be used as a manufacturing facility. The purchase price for the property was €0.6 million, exclusive of any closing costs. |
|
|
(3) |
Volition Diagnostics signed a new 24-month lease for this property located at 93-95 Gloucester Place, London, W1U 6JQ, United Kingdom, commencing February 1, 2022 until January 31, 2024, at an annual rent of £64,800 GBP. |
|
|
(4) |
Singapore Volition signed a one-year lease for this property, commencing July 1, 2021, located at 111 Somerset Road, Level 3, Triple One, Somerset, Singapore 238164, at an annual rent of SGD103,692. |
|
|
(5) |
VolitionRx signed a three-year lease for this property, commencing on June 1, 2019, located at 13215 Bee Cave Parkway, Suite 125, Galleria Oaks B, Austin, Texas 78738, at an annual rent of $34,384. |
ITEM 3. |
LEGAL PROCEEDINGS |
In the ordinary course of business, we may be subject to claims, counter claims, suits and other litigation of the type that generally arise from the conduct of our business. We are not aware of any threatened or pending litigation that we expect will have a material adverse effect on our business operations, financial condition or results of operations.
ITEM 4. |
MINE SAFETY DISCLOSURES |
Not Applicable.
| 28 |
| Table of Contents |
PART II
ITEM 5. |
MARKET FOR THE REGISTRANT’S COMMON EQUITY, RELATED STOCKHOLDER MATTERS AND ISSUER PURCHASES OF EQUITY SECURITIES |
Market Information
Our common stock is traded on the NYSE American under the symbol “VNRX”.
Holders
As of March 25, 2022, there were 53,775,261 shares of our common stock outstanding held by 126 holders of record, based on information provided by our transfer agent. The actual number of stockholders is greater than this number of record holders and includes stockholders who are beneficial owners but whose shares are held in street name by brokers and other nominees.
Dividends
We have not declared or paid any cash dividends on our common stock since inception and presently anticipate that all earnings, if any, will be retained for development of our business and that no dividends on our common stock will be declared in the foreseeable future. Any future dividends will be subject to the discretion of our board of directors and will depend upon, among other things, future earnings, operating and financial conditions, capital requirements, general business conditions and other pertinent facts. Therefore, there can be no assurance that any dividends on our common stock will be paid in the future.
Securities Authorized for Issuance Under Equity Compensation Plans
The information required under this item is incorporated by reference from our definitive proxy statement related to our 2022 Annual Meeting of Stockholders, to be filed pursuant to Regulation 14A, on or before May 2, 2022.
Recent Sales of Unregistered Securities
None.
Repurchase of Equity Securities
No equity securities were repurchased during the fourth quarter of 2021.
ITEM 6. |
RESERVED |
| 29 |
| Table of Contents |
ITEM 7. |
MANAGEMENT’S DISCUSSION AND ANALYSIS OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS |
The following discussion of our financial condition and results of operations should be read together with our consolidated financial statements in Part II within this Report. This discussion includes an analysis of our financial condition and results of operations for the years ended December 31, 2021 and 2020 and year-over-year comparisons between those periods. Certain statements made in this section constitute “forward-looking statements,” which are subject to numerous risks and uncertainties including those described in this section. For additional information, refer to the section entitled “Cautionary Note Regarding Forward-Looking Statements” within this Report.
Company Overview
Volition is a multi-national epigenetics company that applies its Nucleosomics™ platform through its subsidiaries to develop simple, easy to use, cost-effective blood tests to help diagnose and monitor a range of life-altering diseases including certain cancers and diseases associated with NETosis such as sepsis and COVID-19. Our mission is to save lives and improve outcomes for millions of people and animals worldwide. Early diagnosis and monitoring have the potential to not only prolong the life of patients, but also to improve their quality of life.
Our tests are based on the science of Nucleosomics™, which is the practice of identifying and measuring nucleosomes in the bloodstream or other bodily fluid - an indication that disease is present. We are primarily focused on human diagnostics and monitoring but also have a subsidiary focused on animal diagnostics and monitoring.
We have five key pillars of focus: Nu.Q®, Nu.Q® NETs, Nu.Q® Capture, Nu.Q® Discover and Nu.Q® Vet, all of which use the same proprietary Nu.Q® platform to commercialize in different areas.
Our research and development activities are centered in Belgium, with an innovation laboratory in California, and additional offices in Texas, London, and Singapore, we focus on bringing our diagnostic and disease monitoring products to market.
We have identified the specific processes and resources required to achieve the near and medium-term objectives of our business plan, including personnel, facilities, equipment, research and testing materials including antibodies and clinical samples, and the protection of intellectual property. To date, operations have proceeded satisfactorily in relation to our business plan. However, it is possible that some resources will not readily become available in a suitable form or on a timely basis or at an acceptable cost. It is also possible that the results of some processes may not be as expected, and that modifications of procedures and materials may be required. Such events could result in delays to the achievement of the near and medium-term objectives of our business plan, in particular the progression of clinical validation studies and regulatory approval processes for the purpose of bringing products to the IVD market.
Our future as an operating business will depend on our ability to obtain sufficient capital contributions, financing and/or generate revenues as may be required to sustain our operations. Management plans to address the above as needed by: (a) securing additional grant funds; (b) obtaining additional equity or debt financing; (c) granting licenses to third parties in exchange for specified up-front and/or back end payments; and (d) developing and commercializing our products on an accelerated timeline. Management continues to exercise tight cost controls to conserve cash.
Our ability to continue as a going concern is dependent upon our accomplishment of the plans described in the preceding paragraph and eventually to attain profitable operations. The accompanying consolidated financial statements do not include any adjustments that might be necessary if we are unable to continue as a going concern. If we are unable to obtain adequate capital, we could be forced to cease operations.
Developments—COVID-19 Pandemic
Throughout 2020 and 2021, in response to the COVID-19 pandemic we implemented contingency planning to protect the health and well-being of our employees, with the majority of our employees working remotely where possible. We have implemented travel restrictions as well as protocols limiting visitor access to our facilities, and we are following social distancing practices.
As a result of the COVID-19 pandemic, we have experienced and may continue to experience disruptions that could impact our clinical trials, including:
|
· |
delays in enrolling patients in clinical trials; |
|
· |
delays in sample collection; and |
|
· |
diversion of healthcare resources away from the conduct of clinical trials, including the diversion of hospitals serving as clinical trial sites and hospital staff supporting the conduct of our clinical trials. |
| 30 |
| Table of Contents |
The extent to which the COVID-19 pandemic will impact our business, financial condition, and results of operations in the future is highly uncertain and will be affected by a number of factors. These include the duration and extent of the COVID-19 pandemic, the development of new variants of the COVID-19 virus that may be more contagious or virulent than previous versions, the scope of mandated or recommended containment and mitigation measures, the effect of government stabilization and recovery efforts, and the success of vaccine distribution programs.
Liquidity and Capital Resources
We have financed our operations since inception primarily through private placements and public offerings of our common stock. As of December 31, 2021, we had cash and cash equivalents of approximately $20.6 million.
Net cash used in operating activities was $20.9 million and $16.5 million for the years ended December 31, 2021 and December 31, 2020, respectively. The increase in cash used in operating activities during 2021 when compared to 2020 was primarily due to increased payroll costs reflecting growth in staff numbers, higher legal and professional fees in relation to a registered public offering and an increase in marketing expenses.
Net cash used in investing activities was $1.0 million and $1.6 million for the years ended December 31, 2021 and December 31, 2020, respectively. The decrease in cash used in investing activities during 2021 was primarily due to a reduction in purchases of laboratory equipment as compared to 2020.
Net cash provided by financing activities after associated costs was $22.9 million and $20.6 million for the years ended December 31, 2021 and December 31, 2020, respectively. The increase in cash provided by financing activities for the 2021, when compared to 2020 was primarily due to $18.9 million in net cash received from the issuance of shares of common stock in a registered public offering in February 2021, $1.2 million in cash received from the issuance of shares of common stock pursuant to the 2018 Equity Distribution Agreement, $2.7 million in cash received from the issuance of shares of common stock pursuant to the 2020 Equity Distribution Agreement and $0.7 million in cash received from the issuance of shares of common stock pursuant to the 2021 Equity Distribution Agreement compared to $12.7 million in net cash received from the issuance of shares of common stock in a registered public offering in May 2020 and $6.5 million in cash received from the issuance of shares of common stock pursuant to the 2018 Equity Distribution Agreement. For additional information on the “at the market offering program,” refer to Note 7, Common Stock – Equity Distribution Agreements, of the Notes to consolidated financial statements.
The following table summarizes our approximate contractual payments due by year as of December 31, 2021.
Approximate Payments (Including Interest) Due by Year
|
|
Total |
|
|
2022 |
|
|
2023 - 2026 |
|
|
2027 + |
|
||||
Description |
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
||||
Financing lease liabilities |
|
|
636,265 |
|
|
|
62,620 |
|
|
|
244,762 |
|
|
|
328,883 |
|
Operating lease liabilities and short term lease |
|
|
438,452 |
|
|
|
216,850 |
|
|
|
221,602 |
|
|
|
- |
|
Grants repayable |
|
|
296,321 |
|
|
|
44,289 |
|
|
|
108,156 |
|
|
|
143,876 |
|
Long-term debt |
|
|
3,433,450 |
|
|
|
926,743 |
|
|
|
1,893,175 |
|
|
|
613,532 |
|
Collaborative agreements obligations |
|
|
813,501 |
|
|
|
813,501 |
|
|
|
- |
|
|
|
- |
|
Total |
|
|
5,617,989 |
|
|
|
2,064,003 |
|
|
|
2,467,695 |
|
|
|
1,086,291 |
|
We intend to use our cash reserves to predominantly fund further research and development activities. We do not have any substantial source of revenues and expect to rely on additional future financing, through the sale of equity or debt securities, or the sale of licensing or distribution rights, to provide sufficient funding to execute our strategic plan. There is no assurance that we will be successful in raising further funds.
In the event additional financing is delayed, we will prioritize the maintenance of our research and development personnel and facilities, primarily in Belgium, and the maintenance of our patent rights. In such instance, the completion of clinical validation studies and regulatory approval processes for the purpose of bringing products to the IVD market would be delayed. In the event of an ongoing lack of financing, it may be necessary to discontinue operations, which will adversely affect the value of our common stock.
We have not attained profitable operations and are dependent upon obtaining financing to pursue any extensive activities. For these reasons, our auditors included in their report on our audited financial statements for the fiscal year ended December 31, 2021, an explanatory paragraph regarding factors that raise substantial doubt that we will be able to continue as a going concern.
| 31 |
| Table of Contents |
Results of Operations
Comparison of the Years Ended December 31, 2021 and December 31, 2020
The following table sets forth our results of operations for the years ended on December 31, 2021, and December 31, 2020, respectively (expressed in United Stated Dollars, except outstanding share numbers and percentages).
|
|
|
|
|
Increase |
|
|
Percentage Increase |
||||||||
|
|
2021 |
|
|
2020 |
|
|
(Decrease) |
|
|
(Decrease) |
|||||
|
|
$ |
|
|
$ |
|
|
$ |
|
|
% |
|||||
Royalty |
|
|
- |
|
|
|
2,112 |
|
|
|
(2,112 | ) |
|
>100 | % | |
Product |
|
|
90,035 |
|
|
|
11,321 |
|
|
|
78,714 |
|
|
>100 | % | |
Total Revenues |
|
|
90,035 |
|
|
|
13,433 |
|
|
|
76,602 |
|
|
>100 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
15,541,889 |
|
|
|
14,533,862 |
|
|
|
1,008,027 |
|
|
|
7 | % |
General and administrative |
|
|
8,751,392 |
|
|
|
5,654,018 |
|
|
|
3,097,374 |
|
|
|
55 | % |
Sales and marketing |
|
|
4,129,833 |
|
|
|
1,073,368 |
|
|
|
3,056,465 |
|
|
>100 | % | |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Operating Expenses |
|
|
28,423,114 |
|
|
|
21,261,248 |
|
|
|
7,161,866 |
|
|
|
34 | % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Grant income |
|
|
1,522,533 |
|
|
|
635,513 |
|
|
|
887,020 |
|
|
>100 | % | |
(Loss)/Gain on disposal of fixed assets |
|
|
(26,166 | ) |
|
|
293,312 |
|
|
|
319,478 |
|
|
|
<100 | % |
Interest income |
|
|
2,734 |
|
|
|
49,495 |
|
|
|
(46,761 | ) |
|
(94 |
%) | |
Interest expense |
|
|
(155,803 | ) |
|
|
(129,799 | ) |
|
|
26,004 |
|
|
|
20 | % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Total Other Income (Expenses) |
|
|
1,343,298 |
|
|
|
848,521 |
|
|
|
494,777 |
|
|
|
58 | % |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Net Loss |
|
|
(26,989,781 | ) |
|
|
(20,399,294 | ) |
|
|
6,590,487 |
|
|
|
32 | % |
Revenues
Our operations are still predominantly in the research and development stage and we had minimal revenues of $90,035 and $13,433 during the years ended December 31, 2021 and December 31, 2020, respectively. The main source of revenues during the year ended December 31, 2021 was direct sales of the Nu.Q® Vet Cancer Screening Test via the Gastrointestinal Laboratory at Texas A&M University.
Operating Expenses
Total operating expenses increased to $28.4 million from $21.3 million for the years ended December 31, 2021 and December 31, 2020, respectively, as a result of the factors described below.
| 32 |
| Table of Contents |
Research and Development Expenses
Research and development expenses increased to $15.5 million from $14.5 million for the years ended December 31, 2021 and December 31, 2020 respectively. The increase in overall research and development expenditures during 2021 was primarily related to higher personnel expenses and stock-based compensation partially offset by lower research collaboration and antibody costs together with increased laboratory costs. FTE personnel numbers within this division increased by ten to fifty seven during 2021 compared to the prior year period.
|
|
2021 |
|
|
2020 |
|
|
Change |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Personnel expenses |
|
|
6,405,197 |
|
|
|
5,171,967 |
|
|
|
1,233,230 |
|
Stock based compensation |
|
|
1,361,989 |
|
|
|
340,075 |
|
|
|
1,021,914 |
|
Direct research and development expenses |
|
|
5,517,082 |
|
|
|
6,384,169 |
|
|
|
(867,087 | ) |
Other research and development |
|
|
1,288,467 |
|
|
|
1,784,111 |
|
|
|
(495,644 | ) |
Depreciation and amortization |
|
|
969,154 |
|
|
|
853,540 |
|
|
|
115,614 |
|
Total research and development expenses |
|
|
15,541,889 |
|
|
|
14,533,862 |
|
|
|
1,008,027 |
|
General and Administrative Expenses
General and administrative expenses increased to $8.8 million from $5.7 million for the years ended December 31, 2021 and December 31, 2020, respectively. The increase in overall general and administrative expenditures during 2021 was primarily due to higher personnel expenses, stock-based compensation, director and officer liability insurance and legal fees in connection with our capital raises. The FTE personnel number within this division increased by three to thirteen in 2021 compared to the prior year period.
|
|
2021 |
|
|
2020 |
|
|
Change |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Personnel expenses |
|
|
2,723,604 |
|
|
|
2,135,578 |
|
|
|
588,026 |
|
Stock-based compensation |
|
|
2,984,253 |
|
|
|
887,181 |
|
|
|
2,097,072 |
|
Legal and professional fees |
|
|
1,766,377 |
|
|
|
1,611,495 |
|
|
|
154,882 |
|
Other general and administrative |
|
|
1,148,133 |
|
|
|
831,931 |
|
|
|
316,202 |
|
Depreciation and amortization |
|
|
129,025 |
|
|
|
187,833 |
|
|
|
(58,808 | ) |
Total general and administrative expenses |
|
|
8,751,392 |
|
|
|
5,654,018 |
|
|
|
3,097,374 |
|
Sales and Marketing Expenses
Sales and marketing expenses increased to $4.1 million from $1.1 million for the years ended December 31, 2021 and December 31, 2020, respectively. The increase in overall sales and marketing expenditures was primarily due to increased personnel expenses, stock-based compensation and direct marketing expenses. The FTE personnel number within this division increased by ten to thirteen in 2021 compared to the prior year period.
|
|
2021 |
|
|
2020 |
|
|
Change |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Personnel expenses |
|
|
2,354,732 |
|
|
|
545,842 |
|
|
|
1,808,890 |
|
Stock-based compensation |
|
|
774,404 |
|
|
|
164,236 |
|
|
|
610,168 |
|
Direct marketing and professional fees |
|
|
1,000,697 |
|
|
|
363,290 |
|
|
|
637,407 |
|
Total sales and marketing expenses |
|
|
4,129,833 |
|
|
|
1,073,368 |
|
|
|
3,056,465 |
|
| 33 |
| Table of Contents |
Other Income (Expenses)
For the year ended December 31, 2021, other income increased to approximately $1.3 million compared to other income of approximately $0.8 million for the year ended December 31, 2020. This increase in other income was primarily due to grant income received of approximately $1.5 million during 2021.
Net Loss
For the year ended December 31, 2021, the Company’s net loss was $27.0 million, an increase of approximately $6.6 million, in comparison to a net loss of $20.4 million for the year ended December 31, 2020. The change was a result of the factors described above.
Going Concern
We have not attained profitable operations and are dependent upon obtaining external financing to continue to pursue our operational and strategic plans. For these reasons, management has determined that there is substantial doubt that the business will be able to continue as a going concern without further financing.
Off-Balance Sheet Arrangements
We have no significant off-balance sheet arrangements that have or are reasonably likely to have a current or future effect on our financial condition, changes in financial condition, revenues or expenses, results of operations, liquidity, capital expenditures or capital resources that are material to stockholders.
Future Equity or Debt Financings
We may seek to obtain additional capital through the sale of debt or equity securities if we deem it desirable or necessary. These sales may include the sale of equity securities from time to time through our “at the market offering program” with Cantor Fitzgerald & Co. and Oppenheimer and Co. Inc. under the 2021 Equity Distribution Agreement (see Note 7, Common Stock – Equity Distribution Agreements, of the Notes to consolidated financial statements). However, we may be unable to obtain such additional capital when needed, or on terms favorable to us or our stockholders, if at all. If we raise additional funds by issuing equity securities, the percentage ownership of our stockholders will be reduced, stockholders may experience additional dilution, or such equity securities may provide for rights, preferences or privileges senior to those of the holders of our common stock. If additional funds are raised through the issuance of debt securities, the terms of such securities may place restrictions on our ability to operate our business.
Critical Accounting Policies and Estimates
Our consolidated financial statements and accompanying notes have been prepared in accordance with U.S. generally accepted accounting principles, (“U.S. GAAP”), applied on a consistent basis. The preparation of consolidated financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting periods.
We also regularly evaluate estimates and assumptions related to deferred income tax asset valuation allowances, useful lives of property and equipment and intangible assets, borrowing rate used in operating lease right-of-use asset and liability valuations, impairment analysis of intangible assets and valuations of stock-based compensation.
We base our estimates and assumptions on current facts, historical experiences, information from third party professionals and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. Actual results may differ materially and adversely from our estimates. To the extent there are material differences between the estimates and the actual results, future results of operations could be affected.
We regularly evaluate the accounting policies that we use to prepare our consolidated financial statements. A complete summary of these policies is included in the Notes to our consolidated financial statements.
We have determined that for the periods reported in this Report the following accounting policies are critical in understanding our financial condition and results of operations:
| 34 |
| Table of Contents |
Stock-Based Compensation
The Company records stock-based compensation in accordance with ASC 718, “Compensation – Stock Compensation”. Under the provisions of ASC 718, stock-based compensation cost is measured at the grant date, based on the fair value of the award, and is recognized over the employee’s requisite service period, which is generally the vesting period. The fair value of our stock options and warrants is estimated using a Black-Scholes option valuation model. Restricted stock units are valued based on the closing stock price on the date of grant, refer to Note 8 of the consolidated financial statements for further details.
Impairment of Long-Lived Assets
In accordance with ASC 360, “Property Plant and Equipment”, the Company tests long-lived assets or asset groups for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. Circumstances which could trigger a review include, but are not limited to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; and current expectation that the asset will more likely than not be sold or disposed of significantly before the end of its estimated useful life. Recoverability is assessed based on the carrying amount of the asset and its fair value which is generally determined based on the sum of the undiscounted cash flows expected to result from the use and the eventual disposal of the asset, as well as specific appraisal in certain instances. An impairment loss is recognized when the carrying amount is not recoverable and exceeds fair value. Impairment losses of $nil and $nil were recognized during the years ended December 31, 2021 and December 31, 2020, respectively.
Foreign Currency Translation
The Company has functional currencies in Euros, U.S. Dollars and British Pounds Sterling and its reporting currency is the U.S. Dollar. Management has adopted ASC 830-20, “Foreign Currency Matters – Foreign Currency Transactions” All assets and liabilities denominated in foreign currencies are translated using the exchange rate prevailing at the balance sheet date. For revenues and expenses, the weighted average exchange rate for the period is used. Gains and losses arising on translation of foreign currency denominated transactions are included in Other Comprehensive Income.
Recently Issued Accounting Pronouncements
The Company has implemented all applicable new accounting pronouncements that are in effect. The Company does not believe that there are any other applicable new accounting pronouncements that have been issued that might have a material impact on its financial position or results of operations.
ITEM 7A. |
QUANTITATIVE AND QUALITATIVE DISCLOSURES ABOUT MARKET RISK |
We are a smaller reporting company and are not required to disclose this information.
| 35 |
| Table of Contents |
ITEM 8. |
FINANCIAL STATEMENTS AND SUPPLEMENTARY DATA |
VOLITIONRX LIMITED
Consolidated Financial Statements
For the Years Ended December 31, 2021 and 2020
|
|
Index |
|
|
|
|
|
Report of Independent Registered Public Accounting Firm (PCAOB ID: |
|
F - 37 |
|
|
F - 38 |
|
|
Consolidated Statements of Operations and Comprehensive Loss |
|
F - 39 |
|
|
F - 40 |
|
|
|
F - 41 |
|
|
|
F - 42 |
|
| F-36 |
| Table of Contents |
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Shareholders of VolitionRx Limited:
Opinion on the Financial Statements
We have audited the accompanying consolidated balance sheets of VolitionRx Limited (“the Company”) as of December 31, 2021 and 2020, the related consolidated statements of operations and comprehensive loss, stockholders’ equity, and cash flows for each of the years in the two-year period ended December 31, 2021 and the related notes (collectively referred to as the “financial statements”). In our opinion, the financial statements referred to above present fairly, in all material respects, the financial position of the Company as of December 31, 2021 and 2020, and the results of its operations and its cash flows for each of the years in the two-year period ended December 31, 2021, in conformity with accounting principles generally accepted in the United States of America.
Explanatory Paragraph Regarding Going Concern
The accompanying financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 2 to the financial statements, the Company has incurred losses since inception, has negative cash flows from operations, and has minimal revenues, which creates substantial doubt about its ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 2. The financial statements do not include any adjustments that might result from the outcome of this uncertainty.
Basis for Opinion
These financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (“PCAOB”) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining on a test basis, evidence regarding the amounts and disclosures in the financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the financial statements. We believe that our audits provide a reasonable basis for our opinion.
Critical Audit Matters
The critical audit matters communicated below are matters arising from the current period audit of the financial statements that were communicated or required to be communicated to the audit committee and that: (1) related to accounts or disclosures that are material to the financial statements and (2) involved our especially challenging, subjective, or complex judgements. We determined that there are no critical audit matters.
/s/
We have served as the Company’s auditor since 2011.
March 30, 2022
| F-37 |
| Table of Contents |
VOLITIONRX LIMITED
Consolidated Balance Sheets
(Expressed in United States Dollars, except share numbers)
|
|
December 31, |
|
|
December 31, |
|
||
|
|
2021 |
|
|
2020 |
|
||
|
|
$ |
|
|
$ |
|
||
ASSETS |
|
|
|
|
|
|
||
Current Assets |
|
|
|
|
|
|
||
Cash and cash equivalents |
|
|
|
|
|
|
||
Accounts Receivable |
|
|
|
|
|
|
||
Prepaid expenses |
|
|
|
|
|
|
||
Other current assets |
|
|
|
|
|
|
||
Total Current Assets |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Property and equipment, net |
|
|
|
|
|
|
||
Operating lease right-of-use assets |
|
|
|
|
|
|
||
Intangible assets, net |
|
|
|
|
|
|
||
Total Assets |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
LIABILITIES AND STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
Current Liabilities |
|
|
|
|
|
|
|
|
Accounts payable |
|
|
|
|
|
|
||
Accrued liabilities |
|
|
|
|
|
|
||
Management and directors’ fees payable |
|
|
|
|
|
|
||
Current portion of long-term debt |
|
|
|
|
|
|
||
Current portion of financing lease liabilities |
|
|
|
|
|
|
||
Current portion of operating lease liabilities |
|
|
|
|
|
|
||
Current portion of grant repayable |
|
|
|
|
|
|
||
Total Current Liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Long-term debt, net of current portion |
|
|
|
|
|
|
||
Finance lease liabilities, net of current portion |
|
|
|
|
|
|
||
Operating lease liabilities, net of current portion |
|
|
|
|
|
|
||
Grant repayable, net of current portion |
|
|
|
|
|
|
||
Total Liabilities |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
STOCKHOLDERS’ EQUITY |
|
|
|
|
|
|
|
|
Common Stock Authorized: |
|
|
|
|
|
|
||
Additional paid-in capital |
|
|
|
|
|
|
||
Accumulated other comprehensive income (loss) |
|
|
|
|
|
( |
) | |
Accumulated deficit |
|
|
( |
) |
|
|
( |
) |
Total VolitionRx Limited Stockholders’ Equity |
|
|
|
|
|
|
||
Non-controlling interest |
|
|
( |
) |
|
|
( |
) |
Total Stockholders’ Equity |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Total Liabilities and Stockholders’ Equity |
|
|
|
|
|
|
||
(The accompanying notes are an integral part of these consolidated financial statements)
| F-38 |
| Table of Contents |
VOLITIONRX LIMITED
Consolidated Statements of Operations and Comprehensive Loss
(Expressed in United States Dollars, except share numbers)
|
|
For the year ended |
|
|||||
|
|
December 31, 2021 |
|
|
December 31, 2020 |
|
||
|
|
$ |
|
|
$ |
|
||
Revenues |
|
|
|
|
|
|
||
Royalty |
|
|
|
|
|
|
||
Product |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Total Revenues |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Operating Expenses |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Research and development |
|
|
|
|
|
|
||
General and administrative |
|
|
|
|
|
|
||
Sales and marketing |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Total Operating Expenses |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Operating Loss |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
Other Income (Expenses) |
|
|
|
|
|
|
|
|
Grant income |
|
|
|
|
|
|
||
(Loss)/Gain on disposal of fixed assets |
|
|
( |
) |
|
|
|
|
Interest income |
|
|
|
|
|
|
||
Interest expense |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
Total Other Income (Expenses) |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Net Loss |
|
|
( |
) |
|
|
( |
) |
Net Loss attributable to Non-Controlling Interest |
|
|
|
|
|
|
||
Net Loss attributable to VolitionRx Limited Stockholders |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
Other Comprehensive Income (Loss) |
|
|
|
|
|
|
|
|
Foreign currency translation adjustments |
|
|
|
|
|
( |
) | |
Net Comprehensive Loss |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
Net Loss Per Share – Basic and Diluted attributable to VolitionRx Limited Stockholders |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
Weighted Average Shares Outstanding |
|
|
|
|
|
|
|
|
– Basic and Diluted |
|
|
|
|
|
|
||
(The accompanying notes are an integral part of these consolidated financial statements)
| F-39 |
| Table of Contents |
VOLITIONRX LIMITED
Consolidated Statement of Stockholders’ Equity
For the Years Ended December 31, 2021 and 2020
(Expressed in United States Dollars, except share numbers)
|
|
|
|
|
|
|
|
Accumulated Other |
|
|
|
|
|
|
|
|||||||||||||
|
|
Common Stock |
|
|
Additional Paid-in |
|
|
Comprehensive Income |
|
|
Accumulated |
|
|
Non Controlling |
|
|
|
|||||||||||
|
|
Shares |
|
|
Amount |
|
|
Capital |
|
|
(Loss) |
|
|
Deficit |
|
|
Interest |
|
|
Total |
|
|||||||
|
|
# |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|
$ |
|
|||||||
Balance, December 31, 2019 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for Director compensation in Volition Germany |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Common stock repurchase and retirement |
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
( |
) | |||
Common stock issued for exercise of stock options |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Common stock issued for exercise of warrants |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Common stock issued in public offerings, net |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Tax withholdings paid related to stock-based compensation |
|
|
- |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
( |
) | ||||
Stock-based compensation |
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Foreign currency translation |
|
|
- |
|
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
( |
) | ||||
Net loss for the Year |
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) | |||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Balance, December 31, 2020 |
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common stock issued for cashless exercise of stock options |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Common stock issued for settlement of RSUs |
|
|
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Common stock issued in public offerings, net |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
Tax withholdings paid related to stock-based compensation |
|
|
- |
|
|
|
|
|
|
( |
) |
|
|
|
|
|
|
|
|
|
|
|
( |
) | ||||
Stock-based compensation |
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Stock-based compensation in relation to modification of options |
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
Foreign currency translation |
|
|
- |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
- |
|
|
|
|
|||||
Net loss for the Year |
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
- |
|
|
|
( |
) |
|
|
( |
) |
|
|
( |
) |
Balance, December 31, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
( |
) |
|
|
( |
) |
|
|
|
|||||
(The accompanying notes are an integral part of these consolidated financial statements)
| F-40 |
| Table of Contents |
VOLITIONRX LIMITED
Consolidated Statements of Cash Flows
(Expressed in United States Dollars)
|
|
For the year ended |
|
|||||
|
|
December 31, 2021 |
|
|
December 31, 2020 |
|
||
|
|
$ |
|
|
$ |
|
||
Operating Activities: |
|
|
|
|
|
|
||
Net loss |
|
|
( |
) |
|
|
( |
) |
Adjustments to reconcile net loss to net cash used in operating activities: |
|
|
|
|
|
|
|
|
Depreciation and amortization |
|
|
|
|
|
|
||
Amortization of operating lease right-of-use assets |
|
|
|
|
|
|
||
Loss (Gain) on disposal of fixed assets |
|
|
|
|
|
( |
) | |
Stock based compensation |
|
|
|
|
|
|
||
Common stock issued for Director compensation in Volition Germany |
|
|
|
|
|
|
||
Stock-based compensation in relation to modification of options |
|
|
|
|
|
|
||
Changes in operating assets and liabilities: |
|
|
|
|
|
|
|
|
Prepaid expenses |
|
|
( |
) |
|
|
( |
) |
Accounts receivable |
|
|
( |
) |
|
|
( |
) |
Other current assets |
|
|
( |
) |
|
|
( |
) |
Accounts payable and accrued liabilities |
|
|
|
|
|
|
||
Management and directors’ fees payable |
|
|
|
|
|
|
||
Right-of-use assets operating leases liabilities |
|
|
( |
) |
|
|
( |
) |
Net Cash Used In Operating Activities |
|
|
( |
) |
|
|
( |
) |
|
|
|
|
|
|
|
|
|
Investing Activities: |
|
|
|
|
|
|
|
|
Purchases of property and equipment |
|
|
( |
) |
|
|
(1,941,060 | ) |
Proceeds from sales of property and equipment |
|
|
|
|
|
|
||
Net Cash Used In Investing Activities |
|
|
( |
) |
|
|
( |
) |
Financing Activities: |
|
|
|
|
|
|
|
|
Net proceeds from issuance of common shares |
|
|
|
|
|
|
||
Tax withholdings paid related to stock-based compensation |
|
|
( |
) |
|
|
( |
) |
Common stock repurchased |
|
|
|
|
|
( |
) | |
Proceeds from grants repayable |
|
|
|
|
|
|
||
Proceeds from long-term debt |
|
|
|
|
|
|
||
Payments on long-term debt |
|
|
( |
) |
|
|
( |
) |
Payments on grants repayable |
|
|
( |
) |
|
|
( |
) |
Payments on financing leases |
|
|
(58,210 | ) |
|
|
( |
) |
Net Cash Provided By Financing Activities |
|
|
|
|
|
|
||
Effect of foreign exchange on cash and cash equivalents |
|
|
|
|
|
( |
) | |
|
|
|
|
|
|
|
|
|
Net Change in Cash and Cash Equivalents |
|
|
|
|
|
|
||
Cash and Cash Equivalents – Beginning of Year |
|
|
19,444,737 |
|
|
|
|
|
Cash and Cash Equivalents – End of Year |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Supplemental Disclosures of Cash Flow Information: |
|
|
|
|
|
|
|
|
Interest paid |
|
|
|
|
|
|
||
Income tax paid |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Non-Cash Financing Activities: |
|
|
|
|
|
|
|
|
Common Stock issued on exercises of stock options and warrants and settlement of RSUs |
|
|
|
|
|
|
||
Loan payable for purchase of manufacturing building |
|
|
|
|
|
|
||
Offering costs from issuance of common stock |
|
|
|
|
|
|
||
(The accompanying notes are an integral part of these consolidated financial statements)
| F-41 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 1 - Nature of Operations
The Company was incorporated under the laws of the State of Delaware on September 24, 1998. On September 22, 2011, the Company filed a Certificate for Renewal and Revival of Charter with the Secretary of State of Delaware. Pursuant to Section 312(1) of the Delaware General Corporation Law, the Company was revived under the new name of “VolitionRX Limited” and the name change became effective on October 11, 2011. On October 7, 2016, the Company amended its Certificate of Incorporation to reflect a name change to “VolitionRx Limited.”
On October 6, 2011, the Company entered into a share exchange agreement with Singapore Volition Pte. Limited, a Singapore corporation incorporated on August 5, 2010 (“Singapore Volition”), and the shareholders of Singapore Volition. Pursuant to the terms of the share exchange agreement, the former shareholders of Singapore Volition held 85% of the issued and outstanding common shares of the Company. The issuance was deemed to be a reverse acquisition for accounting purposes and as such, Singapore Volition is regarded as the predecessor of the Company. The number of shares outstanding and per share amounts of the Company have been restated to recognize the foregoing recapitalization.
The Company’s principal business objective through its subsidiaries is to develop and bring to market simple, easy to use, cost effective blood tests designed to help diagnose and monitor a range of life-altering diseases, including some cancers and diseases associated with NETosis such as sepsis and COVID-19. The tests are based on the science of NucleosomicsTM, which is the practice of identifying and measuring nucleosomes in the bloodstream or other bodily fluid – an indication that disease is present. The Company has two wholly owned subsidiaries, Volition Global Services SRL (“Volition Global”) which was formed in August 2021 and Singapore Volition. Singapore Volition has one wholly owned subsidiary, Belgian Volition SRL, a Belgium private limited liability company (“Belgian Volition”), which it acquired in September 2010. Belgian Volition has four subsidiaries, Volition Diagnostics UK Limited (“Volition Diagnostics”), which was formed in November 2015, Volition America, Inc. (“Volition America”), which was formed in February 2017, Volition Germany GmbH (“Volition Germany”), which was acquired in January 2020, as well as its majority-owned subsidiary Volition Veterinary Diagnostics Development LLC, (“Volition Vet”), which was formed in June 2019. Following the acquisition of Singapore Volition in 2011, the Company’s fiscal year end was changed from August 31 to December 31.
Note 2 - Going Concern
The Company’s consolidated financial statements are prepared using accounting principles generally accepted in the United States of America (“U.S. GAAP”), applicable to a going concern which contemplates the realization of assets and liquidation of liabilities in the normal course of business. The Company has incurred losses since inception of $
The future of the Company as an operating business will depend on its ability to obtain sufficient capital contributions, financing and/or generate revenues as may be required to sustain its operations. Management plans to address the above as needed by, (a) securing additional grant funds, (b) obtaining additional financing through debt or equity transactions; (c) granting licenses and/or distribution rights to third parties in exchange for specified up-front and/or back-end payments, and (d) developing and commercializing its products on an accelerated timeline. Management continues to exercise tight cost controls to conserve cash.
The ability of the Company to continue as a going concern is dependent upon its ability to successfully accomplish the plans described in the preceding paragraph and to eventually attain profitable operations. The accompanying consolidated financial statements do not include any adjustments that might be necessary if the Company is unable to continue as a going concern. If the Company is unable to obtain adequate capital, it could be forced to cease operations.
| F-42 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies
Basis of Presentation
The consolidated financial statements of the Company have been prepared in accordance with U.S. GAAP and are expressed in US dollars. The Company’s fiscal year end is December 31.
Use of Estimates
The preparation of financial statements in conformity with U.S. GAAP requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities, the disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of revenues and expenses during the reporting period. The Company also regularly evaluates estimates and assumptions related to deferred income tax asset valuation allowances, useful lives of property and equipment and intangible assets, borrowing rate used in operating lease right-of-use asset and liability valuations, impairment analysis of intangible assets and valuations of stock-based compensation.
The Company bases its estimates and assumptions on current facts, historical experiences and various other factors that it believes to be reasonable under the circumstances, the results of which form the basis for making judgments about the carrying values of assets and liabilities and the accrual of costs and expenses that are not readily apparent from other sources. The actual results experienced by the Company may differ materially and adversely from the Company’s estimates. To the extent there are material differences between the estimates and the actual results, future results of operations could be affected.
Principles of Consolidation
The accompanying consolidated financial statements for the year ended December 31, 2021 include the accounts of the Company and its subsidiaries, Singapore Volition, Belgian Volition, Volition Diagnostics UK Limited, Volition Germany, Volition America, Volition Vet, and Volition Global Services SRL. See Note 10(f) for more information regarding Volition Vet, Volition Germany, Volition America and Singapore Volition. All intercompany balances and transactions have been eliminated in consolidation.
Cash and Cash Equivalents
The Company considers all highly liquid instruments with a maturity of three months or less at the time of issuance to be cash equivalents. As of December 31, 2021, and December 31, 2020, the Company had $
Accounts Receivable
Trade accounts receivable are stated at the amount the Company expects to collect. Due to the nature of the accounts receivable balance, the Company believes the risk of doubtful accounts is minimal and therefore no allowance is recorded. If the financial condition of the Company’s customers were to deteriorate, adversely affecting their ability to make payments, additional allowances would be required. The Company may provide for estimated uncollectible amounts through a charge to earnings and a credit to a valuation allowance. Balances that remain outstanding after the Company has used reasonable collection efforts are written off through a charge to the valuation allowance and a credit to accounts receivable. As of December 31, 2021, the accounts receivable balance was $
Property and Equipment
Property and equipment are stated at historical cost and depreciated over the useful life of the asset using the straight-line method. Useful lives are assigned to assets depending on their category. For details regarding property and equipment, refer to Note 4.
| F-43 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies (continued)
Basic and Diluted Net Loss Per Share
The Company computes net loss per share in accordance with Accounting Standards Codification (“ASC”) 260, “Earnings Per Share,” which requires presentation of both basic and diluted earnings per share (“EPS”) on the face of the income statement. Basic EPS is computed by dividing net loss available to common stockholders (numerator) by the weighted average number of shares outstanding (denominator) during the period. Diluted EPS gives effect to all dilutive potential common shares outstanding during the period using the treasury stock method. In computing diluted EPS, the average stock price for the period is used in determining the number of shares assumed to be purchased from the exercise of stock options or warrants. As of December 31, 2021, and December 31, 2020,
Foreign Currency Translation
The Company has functional currencies in Euros, US Dollars and British Pounds Sterling and its reporting currency is the US Dollar. Management has adopted ASC 830-20, “Foreign Currency Matters – Foreign Currency Transactions”. All assets and liabilities denominated in foreign currencies are translated using the exchange rate prevailing at the balance sheet date. For revenues and expenses, the weighted average exchange rate for the period is used. Gains and losses arising on translation of foreign currency denominated transactions are included in other comprehensive income (loss).
Financial Instruments
Pursuant to ASC 820, “Fair Value Measurements and Disclosures,” an entity is required to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 establishes a fair value hierarchy based on the level of independent, objective evidence surrounding the inputs used to measure fair value. A financial instrument’s categorization within the fair value hierarchy is based upon the lowest level of input that is significant to the fair value measurement. ASC 820 prioritizes the inputs into three levels that may be used to measure fair value:
Level 1
Level 1 applies to assets or liabilities for which there are quoted prices in active markets for identical assets or liabilities.
Level 2
Level 2 applies to assets or liabilities for which there are inputs other than quoted prices that are observable for the assets or liabilities such as quoted prices for similar assets or liabilities in active markets; quoted prices for identical assets or liabilities in markets with insufficient volume or infrequent transactions (less active markets); or model-derived valuations in which significant inputs are observable or can be derived principally from, or corroborated by, observable market data.
Level 3
Level 3 applies to assets or liabilities for which there are unobservable inputs to the valuation methodology that are significant to the measurement of the fair value of the assets or liabilities.
The Company’s financial instruments consist principally of cash, accounts payable, accrued liabilities, notes payable, and amounts due to related parties. Pursuant to ASC 820, the fair value of cash is determined based on “Level 1” inputs, which consists of quoted prices in active markets for identical assets. The Company believes that the recorded values of all of our other financial instruments approximate their current fair values because of their nature and respective maturity dates or durations.
Other Comprehensive Income (Loss)
ASC 220, “Other Comprehensive Income/(Loss)”, establishes standards for the reporting and display of other comprehensive loss and its components in the financial statements. As of December 31, 2021, the Company had $
| F-44 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies (continued)
Income Taxes
Potential benefits of income tax losses are not recognized in the accounts until realization is more likely than not. The Company has adopted ASC 740, “Accounting for Income Taxes” as of its inception. Pursuant to ASC 740, the Company is required to compute tax asset benefits for net operating losses carried forward. The potential benefits of net operating losses have not been recognized in these consolidated financial statements because the Company cannot be assured it is more likely than not it will utilize the net operating losses carried forward in future years. Refer to Note 9 for further details.
Revenue Recognition
The Company adopted ASC 606, “Revenue from Contracts with Customers,” effective January 1, 2019. Under ASC 606, the Company recognizes revenues when the customer obtains control of promised goods or services, in an amount that reflects the consideration which the Company expects to receive in exchange for those goods or services. The Company recognizes revenues following the five-step model prescribed under ASC 606: (i) identify contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenues when (or as) the Company satisfies the performance obligation(s).
The Company generates product revenues from the sale of its Nu.Q® Vet Cancer Screening Test, from the sale of nucleosomes, and from the sale of research use only kits. In addition, revenue is received from external third parties for services the Company performs for them in its laboratory.
Revenues, and their respective treatment for financial reporting purposes under ASC 606, are as follows:
Royalty
The Company receives royalty revenues on the net sales recognized during the period in which the revenue is earned, and the amount is determinable from the licensee. These are presented under “Royalty” under the consolidated statements of operations. The Company does not have future performance obligations under this revenue stream. In accordance with ASC 606, the Company records these revenues based on estimates of the net sales that occurred during the relevant period from the licensee. Differences between actual and estimated royalty revenues are adjusted for in the period in which they become known.
Product
The Company includes revenue from product sales recognized during the period in which goods are shipped to third parties, and the amount is deemed collectable from the third parties. These are presented in “Product” in the consolidated statements of operations and comprehensive loss.
Service
The Company includes revenue recognized from laboratory services performed in the Company’s laboratory on behalf of third parties under “Service” under the consolidated statements of operations.
For each development and/or commercialization agreement that results in revenues, the Company identifies all performance obligations, aside from those that are immaterial, which may include a license to intellectual property and know-how, development activities and/or transition activities. In order to determine the transaction price, in addition to any upfront payment, the Company estimates the amount of variable consideration at the outset of the contract either utilizing the expected value or most likely amount method, depending on the facts and circumstances relative to the contract. The Company constrains the estimates of variable consideration such that it is probable that a significant reversal of previously recognized revenue will not occur throughout the life of the contract. When determining if variable consideration should be constrained, management considers whether there are factors outside the Company’s control that could result in a significant reversal of revenue. In making these assessments, the Company considers the likelihood and magnitude of a potential reversal of revenue. These estimates are re-assessed each reporting period as required.
| F-45 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies (continued)
Research and Development
In accordance with ASC 730, the Company follows the policy of expensing its research and development costs in the period in which they are incurred. The Company incurred research and development expenses of $
Impairment of Long-Lived Assets
In accordance with ASC 360, “Property Plant and Equipment”, the Company tests long-lived assets or asset groups for recoverability when events or changes in circumstances indicate that their carrying amount may not be recoverable. Circumstances which could trigger a review include, but are not limited to: significant decreases in the market price of the asset; significant adverse changes in the business climate or legal factors; accumulation of costs significantly in excess of the amount originally expected for the acquisition or construction of the asset; current period cash flow or operating losses combined with a history of losses or a forecast of continuing losses associated with the use of the asset; and current expectation that the asset will more likely than not be sold or disposed of significantly before the end of its estimated useful life. Recoverability is assessed based on the carrying amount of the asset and its fair value which is generally determined based on the sum of the undiscounted cash flows expected to result from the use and the eventual disposal of the asset, as well as specific appraisal in certain instances. An impairment loss is recognized when the carrying amount is not recoverable and exceeds fair value. Impairment losses of $nil and $nil were recognized during the years ended December 31, 2021 and December 31, 2020, respectively.
Stock-Based Compensation
The Company records stock-based compensation in accordance with ASC 718, “Compensation – Stock Compensation”. Under the provisions of ASC 718, stock-based compensation cost is measured at the grant date, based on the fair value of the award, and is recognized over the employee’s requisite service period, which is generally the vesting period. The fair value of our stock options and warrants is estimated using a Black-Scholes option valuation model. Restricted stock units are valued based on the closing stock price on the date of grant. Refer to Note 8 for further details.
Leases
The Company adopted FASB issued Accounting Standards Update No. 2016-02 – Leases (“Topic 842”) as of January 1, 2019, that requires lessees to record the present value of operating lease payments as right-of-use assets and lease liabilities on the balance sheet. See Note 10(b) for discussion of the guidance and the Company’s accounting policy.
Grant Income
The Company receives funding from public bodies for a proportion of the costs of specific projects. Funds are received in line with claims submitted for the agreed expenditure. The Company recognizes grant income once claims submitted are approved and funds are received. General working capital funding received at the commencement of a project is treated as deferred income and is recorded in accrued liabilities until it has been utilized for the expenditure claimed. Funding received that is repayable is shown as a liability.
Recent Accounting Pronouncements
The Company has implemented all new accounting pronouncements that are in effect. The Company does not believe that there are any other new accounting pronouncements that have been issued that might have a material impact on its financial position or results of operations.
| F-46 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 3 - Summary of Significant Accounting Policies (continued)
COVID-19 Pandemic Impact
The extent to which the COVID-19 pandemic will impact the Company’s business, financial condition, and results of operations in the future is highly uncertain and will be affected by a number of factors. These include the duration and extent of the COVID-19 pandemic, the development of new variants of the COVID-19 virus that may be more contagious or virulent than previous versions, the scope of mandated or recommended containment and mitigation measures, the effect of government stabilization and recovery efforts, and the success of vaccine distribution programs.
| F-47 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 4 - Property and Equipment
The Company’s property and equipment consist of the following amounts as of December 31, 2021 and December 31, 2020:
|
|
|
|
|
|
|
December 31, 2021 |
|
||||||
|
|
|
|
|
|
|
Accumulated |
|
|
Net Carrying |
|
|||
|
|
|
|
Cost |
|
|
Depreciation |
|
|
Value |
|
|||
|
|
Useful Life |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Computer hardware and software |
|
|
|
|
|
|
|
|
|
|
||||
Laboratory equipment |
|
|
|
|
|
|
|
|
|
|
||||
Office furniture and equipment |
|
|
|
|
|
|
|
|
|
|
||||
Buildings |
|
|
|
|
|
|
|
|
|
|
||||
Building improvements |
|
|
|
|
|
|
|
|
|
|
||||
Land |
|
Not amortized |
|
|
|
|
|
- |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
December 31, 2020 |
|
||||||
|
|
|
|
|
|
|
Accumulated |
|
|
Net Carrying |
|
|||
|
|
|
|
Cost |
|
|
Depreciation |
|
|
Value |
|
|||
|
|
Useful Life |
|
$ |
|
|
$ |
|
|
$ |
|
|||
Computer hardware and software |
|
|
|
|
|
|
|
|
|
|
||||
Laboratory equipment |
|
|
|
|
|
|
|
|
|
|
||||
Office furniture and equipment |
|
|
|
|
|
|
|
|
|
|
||||
Buildings |
|
|
|
|
|
|
|
|
|
|
||||
Building improvements |
|
|
|
|
|
|
|
|
|
|
||||
Land |
|
Not amortized |
|
|
|
|
|
- |
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
During the year ended December 31, 2021, the total capital expenditure was $
During the years ended December 31, 2021 and December 31, 2020, the Company recognized $
During the year ended December 31, 2020, the Company sold laboratory equipment for cash proceeds of $
| F-48 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 5 - Intangible Assets
The Company’s intangible assets consist of patents, mainly acquired in the acquisition of Belgian Volition. The patents are being amortized over the assets’ estimated useful lives, which range from 8 to 20 years.
|
|
|
|
|
|
December 31, 2021 |
|
|||||
|
|
|
|
|
Accumulated |
|
|
Net Carrying |
|
|||
|
|
Cost |
|
|
Amortization |
|
|
Value |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Patents |
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
December 31, 2020 |
|
|||||
|
|
|
|
|
Accumulated |
|
|
Net Carrying |
|
|||
|
|
Cost |
|
|
Amortization |
|
|
Value |
|
|||
|
|
$ |
|
|
$ |
|
|
$ |
|
|||
Patents |
|
|
|
|
|
|
|
|
|
|||
During the years ended December 31, 2021 and December 31, 2020, the Company recognized $
The Company amortizes the long-lived assets on a straight-line basis with terms ranging from
2022 |
|
$ |
|
|
2023 |
|
$ |
|
|
2024 |
|
$ |
|
|
Total Intangible Assets |
|
$ |
|
The Company periodically reviews its long-lived assets to ensure that their carrying value does not exceed their fair market value. The Company carried out such a review in accordance with ASC 360 as of December 31, 2021. The result of this review confirmed that the ongoing value of the patents was not impaired as of December 31, 2021.
Note 6 - Related Party Transactions
See Note 7 for common stock issued to related parties and Note 8 for stock options, warrants and RSUs issued to related parties. The Company has agreements with related parties for the purchase of products and consultancy services which are accrued under accruals and management and directors’ fees payable (see consolidated balance sheets).
| F-49 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 7 - Common Stock
As of December 31, 2021, the Company was authorized to issue
2021
Stock Option Exercises
During the year ended December 31, 2021 we issued a total of
|
|
Stock Incentive |
|
|
Stock Options |
|
|
Price Per Share |
|
|
Shares Issued |
|
||||
Date |
|
Plan |
|
|
# |
|
|
$ |
|
|
# |
|
||||
January 13 - March 19, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|||||
February 2, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|||||
February 8, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|||||
February 8, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|||||
February 8 - February 9, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|||||
February 8, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
||||
2020
Stock Option Exercises
During the year ended December 31, 2020 we issued a total of
|
|
Stock Incentive |
|
|
Stock Options |
|
|
Price Per Share |
|
|
Shares Issued |
|
|
Proceeds |
|
|||||
Date |
|
Plan |
|
|
# |
|
|
$ |
|
|
# |
|
|
$ |
|
|||||
February 24 - September 2, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
Cashless |
|
||||||
January 7 - August 17, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
Cashless |
|
||||||
August 12, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
January 7 - August 17, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
Cashless |
|
||||||
August 12, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
January 7, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
Cashless |
|
||||||
January 7, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
Cashless |
|
||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
| F-50 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 7 - Common Stock (continued)
2021
RSU Settlements
During the year ended December 31, 2021 we issued a total of
|
|
Restricted Stock Units Vested |
|
|
Price Settled Per Share |
|
|
Shares Issued |
|
|
Shares Withheld for Tax |
|
||||
Date |
|
# |
|
|
$ |
|
|
# |
|
|
# |
|
||||
January 20, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
April 21, 2021 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
2021
Equity Capital Raises
On February 10, 2021, the Company entered into an underwriting agreement with Cantor Fitzgerald & Co (“Cantor”), in connection with an underwritten public offering of
2020
Equity Capital Raises
On May 20, 2020, the Company entered into an underwriting agreement with National Securities Corporation, acting on its own behalf and as representative of the several underwriters, in connection with the public offering, issuance and sale by the Company of
| F-51 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 7 - Common Stock (continued)
2021 and 2020
Equity Distribution Agreements
On September 24, 2021, the Company entered into an equity distribution agreement (the “2021 EDA”) with Cantor and Oppenheimer & Co. Inc. (“Oppenheimer”), to sell shares of its common stock having an aggregate offering price of up to $
On November 10, 2020, the Company entered into an equity distribution agreement (the “2020 EDA”) with Cantor and Oppenheimer to sell shares of its common stock having an aggregate offering price of up to $
On September 7, 2018, the Company entered into an equity distribution agreement (as amended, the “2018 EDA”) with Oppenheimer to sell shares of common stock having an aggregate offering price of up to $
During the year ended December 31, 2021, the Company raised aggregate net proceeds (net of broker’s commissions and fees) of $
2020
Issuances Upon Warrant Exercises
For the year ended December 31, 2021 no warrants were exercised. During the year ended December 31, 2020 a total of
|
|
Warrants Exercised |
|
|
Price Per Share |
|
|
Shares Issued |
|
|
Proceeds |
|
||||
Date |
|
# |
|
|
$ |
|
|
# |
|
|
$ |
|
||||
September 18, 2020 |
|
|
|
|
|
|
|
|
|
|
|
|
||||
| F-52 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 7 - Common Stock (continued)
2020
Stock Issuance for Services
On January 9, 2020,
2020
Stock Repurchase
On January 12, 2020, the Company purchased from its Chief Medical Officer
| F-53 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 8 - Stock-Based Compensation
a) Warrants
The following table summarizes the changes in warrants outstanding of the Company during the years ended December 31, 2021 and December 31, 2020:
|
|
|
|
Weighted Average |
|
|||
|
|
Number of |
|
|
Exercise Price |
|
||
|
|
Warrants |
|
|
$ |
|
||
Outstanding at December 31, 2019 |
|
|
|
|
|
|
||
Granted |
|
|
|
|
|
|
||
Exercised |
|
|
( |
) |
|
|
|
|
Expired |
|
|
( |
) |
|
|
|
|
Outstanding at December 31, 2020 |
|
|
|
|
|
|
||
Granted |
|
|
|
|
|
|
||
Exercised |
|
|
- |
|
|
|
|
|
Expired |
|
|
- |
|
|
|
|
|
Outstanding at December 31, 2021 |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Exercisable at December 31, 2021 |
|
|
|
|
|
|
||
2021
Effective January 1, 2021, the Company granted warrants to purchase
Effective February 1, 2021, the Company granted warrants to purchase
2020
Effective February 26, 2020, the vesting criteria of the remaining installment of a warrant originally granted March 20, 2013 to an officer of the Company, and previously amended, was deemed met pursuant to the approval of the Compensation Committee, resulting in the vesting of the Warrant as to
Effective March 1, 2020, the Company granted warrants to purchase
| F-54 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 8 - Stock-based Compensation (continued)
a) Warrants (continued)
Below is a table summarizing the warrants issued and outstanding as of December 31, 2021. The warrants outstanding have a weighted average price of $3.88 per share and an aggregate weighted average remaining contractual life of 3.96 years. The warrants exercisable have a weighted average price of $2.75 per share.
|
|
|
|
|
|
|
Weighted Average |
|
|
Proceeds to |
|
|||||||
|
|
|
|
|
|
Exercise |
|
|
Remaining |
|
|
Company if |
|
|||||
Number |
|
|
Number |
|
|
Price |
|
|
Contractual |
|
|
Exercised |
|
|||||
Outstanding |
|
|
Exercisable |
|
|
$ |
|
|
Life (Years) |
|
|
$ |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
- |
|
|
|
|
|
|
|
|
|
|
||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||
Stock-based compensation expense related to warrants of $
b) Options
The Company currently has options outstanding under both its 2011 Equity Incentive Plan (the “2011 Plan”) (for option issuances prior to 2016,) and its 2015 Stock Incentive Plan (the “2015 Plan”) (for option issuances commencing in 2016). Effective as of January 1, 2016, no additional awards were or may be made under the 2011 Plan.
The 2015 Plan was adopted by the Board of Directors on August 18, 2015 and approved by the stockholders at an annual meeting held on October 30, 2015. On August 5, 2016, the Board of Directors adopted an amendment to the 2015 Plan to increase the number of shares of common stock available for issuance under such Plan by
On March 27, 2019, the Board of Directors adopted a subsequent amendment to the 2015 Plan to increase the number of shares of common stock available for issuance under such Plan by
| F-55 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 8 - Stock-based Compensation (continued)
b) Options (continued)
The 2015 Plan permits the grant of incentive stock options, non-statutory stock options, restricted stock awards, stock bonus awards, stock appreciation rights, restricted stock units and performance awards. The primary purpose of the 2015 Plan is to enhance the Company’s ability to attract and retain the services of qualified employees, officers, directors, consultants and other service providers upon whose judgment, initiative and efforts the successful conduct and development of the Company’s business largely depends, and to provide additional incentives to such persons or entities to devote their utmost effort and skill to the advancement and betterment of the Company, by providing them an opportunity to participate in the ownership of the Company that is tied to the Company’s performance, thereby giving them an interest in the success and increased value of the Company. The 2015 Plan is administered by the Compensation Committee comprised solely of members of the Board of Directors or by the Board of Directors as a whole.
The following table summarizes the changes in options outstanding of the Company during the years ended December 31, 2021 and December 31, 2020:
|
|
|
|
Weighted Average |
|
|||
|
|
Number of |
|
|
Exercise Price |
|
||
|
|
Options |
|
|
$ |
|
||
Outstanding at December 31, 2019 |
|
|
|
|
|
|
||
Granted |
|
|
|
|
|
|
||
Exercised |
|
|
( |
) |
|
|
|
|
Expired/Cancelled |
|
|
( |
) |
|
|
|
|
Outstanding at December 31, 2020 |
|
|
|
|
|
|
||
Granted |
|
|
|
|
|
|
||
Exercised |
|
|
( |
) |
|
|
|
|
Expired/Cancelled |
|
|
( |
) |
|
|
|
|
Outstanding at December 31, 2021 |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
Exercisable at December 31, 2021 |
|
|
|
|
|
|
||
2021
Effective May 20, 2021, the Company granted stock options to purchase
| F-56 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 8 - Stock-based Compensation (continued)
b) Options (continued)
During the year ended December 31, 2021, the Company modified a total of
The following table summarizes the amendments to the expiration dates of various options approved during the year ended December 31, 2021. Except as otherwise noted, the expiration dates for all options in the table below were extended from six years to ten years from the original date of grant.
Amendment |
|
|
Equity Incentive |
|
|
Stock Options |
|
|
Grant |
|
|
New Expiration |
|
|
Option Expense |
|
||||||
Date |
|
|
Plan |
|
|
# |
|
|
Date |
|
|
Date |
|
|
$ |
|
||||||
(i) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
(ii) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
(ii) |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
||||||
(i) |
The expiration date of these options were extended from five and a half years to ten years from the original date of grant. |
(ii) |
These options were previously amended on December 16, 2019 and amended again on September 21, 2021. |
| F-57 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 8 - Stock-based Compensation (continued)
b) Options (continued)
Effective August 3, 2021, the Company approved the granting of options under the 2015 Stock Incentive Plan vesting upon achievement of certain corporate goals (see additional details in Note 10(h)). Pursuant to this approval, the Company granted stock options to purchase an aggregate of
Effective September 7, 2021, the Company granted stock options to purchase
Effective October 4, 2021, the Company approved the granting of options under the 2015 Plan vesting upon achievement of certain corporate goals (see additional details in Note 10 (h)). Pursuant to this approval the Company granted stock options to purchase
2020
Effective April 13, 2020, the Company granted stock options to purchase
Effective December 1, 2020, the Company granted stock options to purchase
Below is a table summarizing the options issued and outstanding as of December 31, 2021, all of which were issued pursuant to the 2011 Plan (for option issuances prior to 2016) or the 2015 Plan (for option issuances commencing in 2016)and which have a weighted average exercise price of $
| F-58 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 8 - Stock-Based Compensation (continued)
b) Options (continued)
|
|
|
|
|
|
|
Weighted Average |
|
|
Proceeds to |
|
|||||||
|
|
|
|
|
|
Exercise |
|
|
Remaining |
|
|
Company if |
|
|||||
Number |
|
|
Number |
|
|
Price |
|
|
Contractual Life |
|
|
Exercised |
|
|||||
Outstanding |
|
|
Exercisable |
|
|
$ |
|
|
(Years) |
|
|
$ |
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
Stock-based compensation expense related to stock options of $
As of December 31, 2021, an aggregate of
c) Restricted Stock Units (RSUs)
Below is a table summarizing the RSUs issued and outstanding as of December 31, 2021, all of which were issued pursuant to the 2015 Stock Incentive Plan.
|
|
|
|
Weighted Average |
|
|||
|
|
Number of |
|
|
Exercise Price |
|
||
|
|
RSUs |
|
|
$ |
|
||
Outstanding at December 31, 2019 |
|
|
- |
|
|
|
||
Granted |
|
|
|
|
|
|
||
Outstanding at December 31, 2020 |
|
|
|
|
|
|
||
Granted |
|
|
|
|
|
|
||
Vested |
|
|
( |
) |
|
|
|
|
Cancelled |
|
|
( |
) |
|
|
|
|
Outstanding at December 31, 2021 |
|
|
|
|
|
|
||
| F-59 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 8 - Stock-Based Compensation (continued)
c) Restricted Stock Units (RSUs) (continued)
2021
Effective January 1, 2021, the Company granted RSUs of
Effective March 25, 2021, the Company granted aggregate RSUs of
On March 25, 2021,
On April 13, 2021, 26,250 RSUs vested and resulted in the issuance of
Effective May 1, 2021, the Company granted RSUs of
Effective August 3, 2021, the Company approved the granting of RSUs under the 2015 Plan vesting upon achievement of certain corporate goals (see additional details in Note 10(h)). Pursuant to this approval, the Company granted RSUs of
Effective September 7, 2021, the Company granted RSUs of
Effective October 4, 2021, the Company approved the granting of RSUs under the 2015 Plan vesting upon achievement of certain corporate goals (see additional details in Note 10 (h)). Pursuant to this approval, the Company granted RSUs of
Effective November 1, 2021, the Company granted RSUs of
Effective December 15, 2021, the Company granted RSUs of
| F-60 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 8 - Stock-Based Compensation (continued)
c) Restricted Stock Units (RSUs) (continued)
2020
Effective April 13, 2020, the Company granted RSUs of
Effective December 1, 2020, the Company granted RSUs of
Below is a table summarizing the RSUs issued and outstanding as of December 31, 2021 of which the last to vest have a remaining contractual life of
|
|
|
|
|
Weighted Average |
|
||||
|
|
|
|
|
|
Remaining |
|
|||
Number |
|
|
Share Price |
|
|
Contractual Life |
|
|||
Outstanding |
|
|
$ |
|
|
(Years) |
|
|||
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|||
|
|
|
|
|
|
|
|
|||
Stock-based compensation expense related to RSUs of $
| F-61 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 9 - Income Taxes
The Company has estimated net operating losses carry-forward for the years ended December 31, 2021 and 2020 of $
The significant components of deferred income taxes and assets as of December 31, 2021 and December 31, 2020 are as follows:
|
|
December 31, 2021 |
|
|
December 31, 2020 |
|
||
Net Deferred Tax Asset |
|
$ |
|
|
$ |
|
||
Excess of tax over book depreciation and amortization |
|
|
( |
) |
|
|
( |
) |
ROU Asset |
|
|
( |
) |
|
|
( |
) |
Lease Liability |
|
|
|
|
|
|
||
Accrued expenses |
|
|
|
|
|
|
||
Unrealized Gain/Loss |
|
|
|
|
|
|
||
Stock-based compensation |
|
|
|
|
|
|
||
Net Operating Losses carry-forward |
|
|
|
|
|
|
||
Research and development tax credits |
|
|
|
|
|
|
||
Gross deferred tax assets |
|
|
|
|
|
|
||
Valuation allowance |
|
|
( |
) |
|
|
( |
) |
Net deferred tax asset |
|
|
|
|
|
|
||
Change in Valuation Allowance |
|
|
( |
) |
|
|
|
|
|
|
December 31, 2021 |
|
|
December 31, 2020 |
|
||
Summary Rate Reconciliation |
|
% |
|
|
% |
|
||
Federal statutory rate |
|
|
|
|
|
|
||
State income taxes, net of federal benefit |
|
|
- |
|
|
|
- |
|
Permanent Differences |
|
|
( |
) |
|
|
|
|
Stock based compensation |
|
|
( |
) |
|
|
( |
) |
Federal Research & Development Credits |
|
|
|
|
|
|
||
Foreign taxes |
|
|
|
|
|
|
||
Federal Deferred Rate Decrease |
|
|
( |
) |
|
|
- |
|
Change in Valuation Allowance |
|
|
( |
) |
|
|
( |
) |
Total |
|
|
- |
|
|
|
- |
|
Disclosure Amounts |
|
December 31, 2021 $ |
|
|
|
|
|
|
|
Net Operating Losses - United States |
|
|
|
|
Net Operating Losses - Foreign |
|
|
|
|
Credit Carryforward - United States |
|
|
|
|
Credit Carryforward - Foreign |
|
|
|
|
Increase in Valuation Allowance |
|
|
|
|
| F-62 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 10 - Commitments and Contingencies
a) Finance Lease Obligations
In 2015, the Company entered into an equipment finance lease to purchase three Tecan machines (automated liquid handling robots) for €
In 2016, the Company entered into a real estate capital lease with ING Asset Finance Belgium S.A. (“ING”) to purchase a property located in Belgium for €
In 2018, the Company entered into a capital lease with BNP Paribas leasing solutions to purchase a freezer for the Belgium facility for €25,000, maturing January 2022, with implicit interest of
2022 |
|
$ |
|
|
2023 |
|
$ |
|
|
2024 |
|
$ |
|
|
2025 |
|
$ |
|
|
2026 |
|
$ |
|
|
Greater than 5 years |
|
$ |
|
|
Total |
|
$ |
|
|
Less: Amount representing interest |
|
$ | ( |
) |
Present value of minimum lease payments |
|
$ |
|
b) Operating Lease Right-of-Use Liabilities
The Company adopted Topic 842 on January 1, 2019. The Company elected to adopt this standard using the optional modified retrospective transition method and recognized a cumulative-effect adjustment to the consolidated balance sheet on the date of adoption. Comparative periods have not been restated. With the adoption of Topic 842, the Company’s consolidated balance sheet now contains the following line items: Operating lease right-of-use assets, current portion of operating lease liabilities and operating lease liabilities, net of current portion.
As all the existing leases subject to the new lease standard were previously classified as operating leases by the Company, they were similarly classified as operating leases under the new standard. The Company has determined that the identified operating leases did not contain non-lease components and require no further allocation of the total lease cost. Additionally, the agreements in place did not contain information to determine the rate implicit in the leases, so we used our incremental borrowing rate as the discount rate. Our weighted average discount rate is
As of December 31, 2021, operating lease right-of-use assets and liabilities arising from operating leases were $
| F-63 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 10 – Commitments and Contingencies (continued)
b) Operating Lease Right-of-Use Liabilities (continued)
The following is a schedule showing the future minimum lease payments under operating leases by years and the present value of the minimum payments as of December 31, 2021.
2022 |
|
$ |
|
|
2023 |
|
$ |
|
|
2024 |
|
$ |
|
|
2025 |
|
$ |
|
|
Total Operating Lease Obligations |
|
$ |
|
|
Less: Amount representing interest |
|
$ | ( |
) |
Present Value of minimum lease payments |
|
$ |
|
The Company’s office space leases are short term, and the Company has elected under the short-term recognition exemption not to recognize them on the balance sheet. During the year ended December 31, 2021, $
2022 |
|
$ |
|
|
Total Operating Lease Liabilities |
|
$ |
|
c) Grants Repayable
In 2010, the Company entered into an agreement with the Walloon Region government in Belgium for a colorectal cancer research grant for €
In 2018, the Company entered into an agreement with the Walloon Region government in Belgium for a colorectal cancer research grant for €
In 2020, the Company entered into an agreement with the Walloon Region government in Belgium for a research grant for €
| F-64 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 10 – Commitments and Contingencies (continued)
c) Grants Repayable (continued)
In 2020, the Company entered into an agreement with the Walloon Region government in Belgium for a research grant for €929,433. Per the terms of the agreement, €278,830 of the grant is to be repaid by instalments over 15 years commencing in 2022. In the event that the Company receives revenue from products or services as defined in the agreement, it is due to pay a 4.34% royalty on such revenue to the Walloon Region. The maximum amount payable to the Walloon Region, in respect of the aggregate of the amount repayable of €278,830 and the 4.34% royalty on revenue, is equal to the amount of funding received. As of December 31, 2021, the grant balance repayable was $
As of December 31, 2021, the balance repayable was $
2022 |
|
$ |
|
|
2023 |
|
$ |
|
|
2024 |
|
$ |
|
|
2025 |
|
$ |
|
|
2026 |
|
$ |
|
|
Greater than 5 years |
|
$ |
|
|
Total Grants Repayable |
|
$ |
|
d) Long-Term Debt
In 2016, the Company entered into a
In 2016, the Company entered into a
In 2017, the Company entered into a 4-year loan agreement with Namur Invest for €
In 2017, the Company entered into a 7-year loan agreement with SOFINEX for up to €
In 2018, the Company entered into a 4-year loan agreement with Namur Innovation and Growth for €
In 2019, the Company entered into a
On October 13, 2020, the Company entered into a
On November 23, 2021, the Company entered into a 3 ½ year loan agreement with SOFINEX for a maximum of €
| F-65 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 10 – Commitments and Contingencies (continued)
d) Long-Term Debt (continued)
As of December 31, 2021, the total balance for long-term debt payable was $3,068,622 and the payments remaining were as follows:
2022 |
|
$ |
|
|
2023 |
|
$ |
|
|
2024 |
|
$ |
|
|
2025 |
|
$ |
|
|
2026 |
|
$ |
|
|
Greater than 5 years |
|
$ |
|
|
Total |
|
$ |
|
|
Less: Amount representing interest |
|
$ | ( |
) |
Total Long-Term Debt |
|
$ |
|
e) Collaborative Agreement Obligations
In 2016, the Company entered into a research co-operation agreement with DKFZ, in Germany for a
In 2017, the Company entered into a clinical study research agreement with the University of Michigan for a
In 2018, the
In 2019, the Company entered into a funded sponsored research agreement with the Texas A&M University (“TAMU”) in consideration for the license granted to the Company for a
On September 16, 2020, the Company entered into a research agreement for the bioinformatic analysis of cell-free DNA fragments from whole-genome sequencing with the Hebrew University of Jerusalem for 6 months for a cost to the Company of €
As of December 31, 2021, the total amount to be paid for future research and collaboration commitments was approximately $
2022 |
|
$ |
|
|
Total Collaborative Agreement Obligation |
|
$ |
|
| F-66 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 10 – Commitments and Contingencies (continued)
f) Other Commitments
Volition Vet
On October 25, 2019, the Company entered into an agreement with TAMU for provision of in-kind services of personnel, animal samples and laboratory equipment in exchange for a non-controlling interest of
Volition Germany
On January 10, 2020, the Company, through its wholly-owned subsidiary Belgian Volition, acquired an epigenetic reagent company, Octamer GmbH (“Octamer”), based in Munich, Germany, and hired its founder for his expertise and knowledge to be passed to Company personnel. On March 9, 2020, Octamer was renamed to Volition Germany GmbH (or “Volition Germany”).
Upon considering the definition of a business, as defined in ASC 805 “Business Combinations,” paragraph 805-10-20, which is an integrated set of activities and assets that is capable of being conducted and managed for the purpose of providing a return, the Company has determined that this did not constitute a business. This is primarily due to the fact that additional inputs are needed in the form of training personnel further to produce outputs. Accordingly, the Company has treated this transaction as the hiring of a member of management, described below, rather than accounting for the transaction as a business combination.
The Company agreed to terms of the transaction on December 13, 2019 and closed on January 10, 2020. Pursuant to the transaction agreement, the Company purchased all outstanding shares of Octamer. In exchange, the Company agreed to issue
| F-67 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 10 – Commitments and Contingencies (continued)
f) Other Commitments (continued)
In connection with the transaction agreement, the Company also entered into a 2-year Managing Director’s agreement with the founder of Octamer to continue to manage Volition Germany for a payment of €288,000 payable in equal monthly installments over such 2-year period and a royalty agreement with the founder providing for the payment of royalties in the amount of 6% of net sales of Volition Germany’s nucleosomes as reagents to pharmaceutical companies for use in the development, manufacture and screening of molecules for use as therapeutic drugs for a period of 5 years post-closing.
The Company recorded approximately $
Volition America
On November 3, 2020, the Company entered into a professional services master agreement with Diagnostic Oncology CRO, LLC to conduct a pivotal clinical trial and provide regulatory submission and reimbursement related services. Under the terms of the agreement Diagnostic Oncology CRO, LLC will provide ad hoc consulting assistance on a project-by-project basis related to the review and assessment of existing data and information to prepare recommended intended use claims and coverage/reimbursement plans to support the preparation of FDA pre-submissions, clinical trial protocol development and study administration, and potential 510k regulatory marketing submissions of the Company’s diagnostic tests, including those proposed for use as an adjunct diagnostic tool for common and aggressive forms of Non-Hodgkin’s Lymphoma. The initial projects contemplated by the agreement relating to Non-Hodgkin’s Lymphoma obligate the Company to pay in aggregate of up to $2.9 million over a period of 22 months. Such payment obligations are on a project-by-project basis as deliverables are executed and subject to certain terms and conditions. Additionally, the Company may terminate the agreement or any project with or without cause upon at least 30 days’ prior written notice. Unless earlier terminated, the term of the agreement is until December 31, 2025 or such later date as when all projects have been completed. As of December 31, 2021, $13,738 is currently payable by Company under this agreement.
Singapore Volition
On November 10, 2020, the Company entered into a consulting services agreement through a related party transaction between its wholly owned subsidiary, Singapore Volition and PB Commodities Pte Ltd (“PB Commodities”). This agreement is effective December 1, 2020 and provides for consultancy services to be rendered by Cameron Reynolds through PB Commodities to Singapore Volition. Singapore Volition will also make available the services of Mr. Reynolds, as Group Chief Executive Officer, to the Company and its subsidiaries, pursuant to services agreements entered into by and between Singapore Volition and the Company or its subsidiaries. The term of the agreement is perpetual, commencing on December 1, 2020 until terminated upon six months’ prior notice. The agreement includes a six-month non-compete following termination of the agreement. PB Commodities will receive a monthly fee of $
g) Legal Proceedings
There are no legal proceedings which the Company believes will have a material adverse effect on its financial position.
h) Commitments in Respect of Corporate Goals and Performance-Based Awards
In August 2021, an incentive plan was authorized by the Compensation Committee of the Board of Directors in order to provide company personnel with an element of performance-based compensation tied to the timely achievement of certain corporate goals focused around product development and commercialization.
Effective August 3, 2021, the Company approved an incentive plan to issue equity-based awards and cash bonuses, vesting upon achievement of certain corporate goals, to various personnel including directors, executives, members of management, consultants and employees of the Company and/or its subsidiaries.
Conditional upon the achievement by December 31, 2021 of a specified corporate goal as set forth in the minutes of the Compensation Committee dated August 3, 2021, as well as continued service by the award recipient, the Company at the sole discretion of the Chief Executive Officer and the Chief Financial Officer shall pay a cash bonus to such award recipient). The Company estimates the total compensation expense based on current recipients to be $330,788. As of December 31, 2021, the Company has accrued compensation expense of $330,788 based on the probable outcomes related to the prescribed performance targets.
Conditional upon the achievement by July 1, 2022 of all specified corporate goals as set forth in the minutes of the Compensation Committee dated August 3, 2021, as well as continued service by the award recipient, the Company at the sole discretion of the Chief Executive Officer and the Chief Financial Officer would pay an additional cash bonus to such award recipient. The Company estimates the total compensation expense based on current recipients to be $182,131. As of the December 31, 2021, the Company has accrued compensation expense of $90,403 based on the probable outcomes related to the prescribed performance targets.
As discussed in detail in Note 8, in August and October 2021, a total of 1,000,000 stock options were issued under this plan and 500,000 RSUs were issued under this plan.
As of the December 31, 2021, the Company has recognized compensation expense of $584,044 in relation to stock options and $513,651 in relation to RSUs, based on the probable outcomes related to the prescribed performance targets on the outstanding awards.
| F-68 |
| Table of Contents |
VOLITIONRX LIMITED
Notes to Consolidated Financial Statements
For Years Ended December 31, 2021 and 2020
($ expressed in United States Dollars)
Note 11 - Subsequent Events
Common Stock Issuances, Option Exercises and RSU Grants
Effective February 8, 2022, the Company granted RSUs of
Effective March 1, 2022, the Company granted RSUs of
Equity Distribution Agreements
From January 1, 2022 to March 10, 2022, the Company raised aggregate net proceeds (net of broker’s commissions and fees) of approximately $
Other Commitments
Effective January 13, 2022, the Company entered into a lease agreement with Aro Partners, a California Limited Partnership for a property of
Other Information
On March 28, 2022, Belgian Volition entered into a License and Supply Agreement (the “License Agreement”) with Heska Corporation (“Heska”), pursuant to which Belgian Volition granted Heska worldwide exclusive rights to sell the NuQ® Vet Cancer Screening Test for companion animals, including dogs and cats, at the point of care (“POC”) and non-exclusive rights to sell its NuQ® Vet Cancer Screening Test through Heska’s central reference laboratories (“Central Lab”). Under the terms of the License Agreement, Belgian Volition will receive an upfront payment of $
END NOTES TO FINANCIALS
| F-69 |
| Table of Contents |
ITEM 9. |
CHANGES IN AND DISAGREEMENTS WITH ACCOUNTANTS ON ACCOUNTING AND FINANCIAL DISCLOSURE |
None.
ITEM 9A. |
CONTROLS AND PROCEDURES |
Disclosure Controls and Procedures
Disclosure controls and procedures are controls and procedures that are designed to ensure that information required to be disclosed in our reports filed under the Exchange Act is recorded, processed, summarized and reported, within the time periods specified in the SEC’s rules and forms. Disclosure controls and procedures include, without limitation, controls and procedures designed to ensure that information required to be disclosed by our company in the reports that it files or submits under the Exchange Act is accumulated and communicated to our management, including our Principal Executive and Principal Financial Officers, or persons performing similar functions, as appropriate to allow timely decisions regarding required disclosure.
Our management carried out an evaluation under the supervision and with the participation of our Principal Executive Officer and Principal Financial Officer, of the effectiveness of the design and operation of our disclosure controls and procedures (as such term is defined in Rules 13a-15(e) and 15d-15(e) under the Exchange Act). Based upon that evaluation, our Principal Executive Officer and Principal Financial Officer have concluded that, as of December 31, 2021, our disclosure controls and procedures were not effective because of material weakness in our internal control over financial reporting relating to the segregation of duties in some areas of finance.
Management’s Report on Internal Control Over Financial Reporting
Management is responsible for establishing and maintaining adequate internal control over financial reporting, as defined in Exchange Act Rule 13a-15(f). The Company’s internal control over financial reporting is a process designed to provide reasonable assurance regarding the reliability of financial reporting and the preparation of financial statements for external purposes in accordance with accounting principles U.S GAAP.
Because of its inherent limitations, internal control over financial reporting may not prevent or detect misstatements. Also, projections of any evaluation of effectiveness to future periods are subject to the risk that controls may become inadequate because of changes in conditions, or that the degree of compliance with the policies or procedures may deteriorate.
Under the supervision and with the participation of management, including the Principal Executive Officer and Principal Financial Officer, the Company conducted an evaluation of the effectiveness of the Company’s internal control over financial reporting as of December 31, 2021, using the criteria established in “Internal Control - Integrated Framework” issued by the Committee of Sponsoring Organizations of the Treadway Commission (“COSO”).
A material weakness is a deficiency, or combination of deficiencies, in internal control over financial reporting, such that there is a reasonable possibility that a material misstatement of the Company’s annual or interim financial statements will not be prevented or detected on a timely basis.
During the year ended December 31, 2021, our management, with oversight from our audit committee, implemented the following remediation steps to address and mitigate all but one of the underlying deficiencies which gave rise to the previously disclosed material weaknesses and to improve our internal control over financial reporting. We anticipate the remaining weakness regarding segregation of duties in some areas of finance to be resolved in 2022.
Remediation steps undertaken include:
Oversight in Information Technologies
|
· |
ensured that third party support and back up is available as cover for our information technology manager; |
|
· |
ensured that appropriate finance approvals are taken before adding users or access for financial systems and applications; and |
|
· |
implemented a quarterly user access control review process across finance and information technology systems. |
| 70 |
| Table of Contents |
As a result of these actions, management has concluded that this particular deficiency has been remedied.
Monitoring of Review Controls with Respect to Accounting for Complex Transactions
|
· |
reallocated responsibilities across the finance organization to ensure that the appropriate level of knowledge and experience is applied based on complexity of tasks being undertaken; |
|
· |
further embedded the use of Certent, an equity management platform, to help with control and reporting of equity awards; |
|
· |
implemented additional review procedures; and |
|
· |
in the event we encounter or anticipate any new and particularly complex transaction we will engage advisors from our wide professional network. |
As a result of these actions, management has concluded that this particular deficiency has been remedied.
Segregation of Duties in Some Areas of Finance
|
· |
hired an additional full-time Business Controller in Belgium with an appropriate level of experience; |
|
· |
hired an experienced financial planning and analysis manager to implement forecasting and budgeting processes; |
|
· |
changed organizational reporting lines and reallocated certain responsibilities to improve segregation of duties; and |
|
· |
implemented additional review procedures at each month end close. |
During 2022, we intend to take additional measures around certain processes we have identified which we believe once implemented and in conjunction with the completed actions above will mitigate and remedy this weakness.
We also intend to take additional steps to further strengthen the control environment. Such measures include but may not be limited to:
|
· |
recruitment of a specialist in Human Resources to recommend and implement relevant policies and processes that will strengthen the control environment; |
|
· |
further strengthening our internal processes and reviews, including formal documentation thereof; |
|
· |
preparation of risk-control matrices to identify key risks and develop and document policies to mitigate those risks; and |
|
· |
engaging additional resources if necessary to help us assess, document, design and implement control activities related to internal control over financial reporting. |
As we continue to evaluate and test the remediation plan outlined above, we may also identify additional measures to address the material weaknesses or modify certain of the remediation procedures described above. We also may implement additional changes to our internal control over financial reporting as may be appropriate in the course of remediating the material weakness. Management, with the oversight of our audit committee, will continue to take steps necessary to remedy the material weakness to reinforce the overall design and capability of our control environment.
In its assessment of the effectiveness of internal control over financial reporting as of December 31, 2021, the Company determined that there were control deficiencies in the segregation of duties in some areas of Finance that constituted a material weakness.
Accordingly, the Company concluded that these control deficiencies resulted in a possibility that a material misstatement of the annual or interim financial statements will not be prevented or detected on a timely basis.
As a result, management has concluded that the Company did not maintain effective internal control over financial reporting as of December 31, 2021, based on criteria established in Internal Control—Integrated Framework issued by COSO.
| 71 |
| Table of Contents |
Changes in Internal Control over Financial Reporting
The Audit Committee of the Board of Directors meets regularly with our financial management, and with the independent registered public accounting firm engaged by us. Internal accounting controls and the quality of financial reporting are discussed during these meetings. The Audit Committee has discussed with the independent registered public accounting firm matters required to be discussed by the auditing standards adopted or established by the Public Company Accounting Oversight Board (“PCAOB”). In addition, the Audit Committee and the independent registered public accounting firm have discussed the independent registered public accounting firm’s independence from the Company and its management, including the matters in the written disclosures required by PCAOB Rule 3526 “Communicating with Audit Committees Concerning Independence.”
There have been no changes in our internal control over financial reporting that occurred during the quarter ended December 31, 2021, that have materially affected, or are reasonably likely to materially affect, our internal control over financial reporting, other than those described above.
The Company is not required to include, and does not include an auditor’s attestation report under SEC Rules. Consequently, the Company’s registered public accounting firm has not attested to management’s reports on the Company’s internal control over financial reporting.
ITEM 9B. |
OTHER INFORMATION |
None.
ITEM 9C. |
DISCLOSURE REGARDING FOREIGN JURISDICTIONS THAT PREVENT INSPECTIONS |
None.
| 72 |
| Table of Contents |
PART III
ITEM 10. |
DIRECTORS, EXECUTIVE OFFICERS AND CORPORATE GOVERNANCE |
The information required under this item is incorporated by reference from our definitive proxy statement related to our 2022 Annual Meeting of Stockholders, or the Proxy Statement, to be filed pursuant to Regulation 14A, on or before May 2, 2022.
ITEM 11. |
EXECUTIVE COMPENSATION |
The information required under this item is incorporated herein by reference from the Proxy Statement.
ITEM 12. |
SECURITY OWNERSHIP OF CERTAIN BENEFICIAL OWNERS AND MANAGEMENT AND RELATED STOCKHOLDER MATTERS. |
The information required under this item is incorporated herein by reference from the Proxy Statement.
ITEM 13. |
CERTAIN RELATIONSHIPS AND RELATED TRANSACTIONS, AND DIRECTOR INDEPENDENCE. |
The information required under this item is incorporated herein by reference from the Proxy Statement.
ITEM 14. |
PRINCIPAL ACCOUNTANT FEES AND SERVICES |
The information required under this item is incorporated herein by reference from the Proxy Statement.
| 73 |
| Table of Contents |
PART IV
ITEM 15. |
EXHIBITS, FINANCIAL STATEMENT SCHEDULES |
|
(a) |
The following documents are filed as part of this Report: |
|
|
|
|
1. |
Financial Statements. Included in Part II, Item 8 of this Report and are incorporated by reference herein. |
|
|
|
|
2. |
Financial Statement Schedules. Financial statement schedules are omitted because they are not applicable, or the required information is shown in the financial statements or notes thereto. |
|
|
|
|
3. |
Exhibits. |
|
|
|
|
Incorporated by Reference |
|
|
||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed Herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Share Purchase Agreement by and between Singapore Volition and ValiRX dated September 22, 2010. |
|
8-K/A |
|
000-30402 |
|
2.1 |
|
5/8/12 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8-K/A |
|
000-30402 |
|
10.15 |
|
1/11/12 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8-K |
|
000-30402 |
|
2.1 |
|
9/29/11 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8-K/A |
|
000-30402 |
|
10.28 |
|
4/5/12 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Second Amended and Restated Certificate of Incorporation, as currently in effect. |
|
8-K |
|
001-36833 |
|
3.1 |
|
10/11/16 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S-8 |
|
333-208512 |
|
4.2 |
|
12/11/15 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-K |
|
001-36833 |
|
4.1 |
|
02/20/20 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8-K/A |
|
000-30402 |
|
10.6 |
|
2/24/12 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employment Agreement by and between VolitionRx and Jason Terrell MD, dated December 29, 2015. |
|
10-K |
|
001-36833 |
|
10.24 |
|
3/11/16 |
|
|
|
| 74 |
| Table of Contents |
|
|
|
|
Incorporated by Reference |
|
|
||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed Herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8-K |
|
000-30402 |
|
4.1 |
|
11/18/11 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8-K |
|
000-30402 |
|
4.2 |
|
11/18/11 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Form Stock Award Agreement for Restricted Stock under the 2011 Equity Incentive Plan. |
|
8-K |
|
000-30402 |
|
4.3 |
|
11/18/11 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8-K |
|
001-36833 |
|
10.1 |
|
06/22/21 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Form of Notice of Stock Option Grant and Stock Option Agreement under the 2015 Stock Incentive Plan. |
|
S-8 |
|
333-214118 |
|
10.2 |
|
10/14/16 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S-8 |
|
333-214118 |
|
10.3 |
|
10/14/16 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S-8 |
|
333-214118 |
|
10.4 |
|
10/14/16 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S-8 |
|
333-214118 |
|
10.5 |
|
10/14/16 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S-8 |
|
333-214118 |
|
10.6 |
|
10/14/16 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S-8 |
|
333-214118 |
|
10.7 |
|
10/14/16 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-Q |
|
001-36833 |
|
10.33 |
|
5/12/15 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8-K |
|
001-36833 |
|
10.1 |
|
10/31/16 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8-K |
|
001-36833 |
|
10.2 |
|
10/31/16 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-K |
|
001-36833 |
|
10.28 |
|
03/10/17 |
|
|
||
| 75 |
| Table of Contents |
|
|
|
|
Incorporated by Reference |
|
|
||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed Herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employment Agreement by and between Volition Diagnostics UK and Martin Faulkes, dated March 7, 2017. |
|
10-K |
|
001-36833 |
|
10.30 |
|
03/10/17 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
8-K |
|
001-36833 |
|
10.1 |
|
09/21/17 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-Q |
|
001-36833 |
|
10.1 |
|
11/09/17 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-K |
|
001-36833 |
|
10.22 |
|
02/20/20 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S-3 |
|
333-236335 |
|
4.3 |
|
2/7/20 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-K |
|
001-36833 |
|
10.15 |
|
03/22/21 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-Q |
|
001-36833 |
|
1.1 |
|
11/12/20 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-Q |
|
001-36833 |
|
10.1 |
|
11/12/20 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Common Stock Warrant issued by VolitionRx to Gael Forterre, dated January 1, 2021. |
|
10-K |
|
001-36833 |
|
10.18 |
|
03/22/21 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-K |
|
001-36833 |
|
10.19 |
|
03/22/21 |
|
|
||
| 76 |
| Table of Contents |
|
|
|
|
Incorporated by Reference |
|
|
||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed Herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-K |
|
001-36833 |
|
10.20 |
|
03/22/21 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-Q |
|
001-36833 |
|
10.7 |
|
05/11/21 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-Q |
|
001-36833 |
|
10.6 |
|
05/11/21 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
S-3 |
|
333-259783 |
|
1.2 |
|
09/24/21 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employment Agreement between Volition America and Gaetan Michel, dated September 15, 2021. |
|
10-Q |
|
001-36833 |
|
10.1 |
|
11/10/21 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
10-Q |
|
001-36833 |
|
10.2 |
|
11/10/21 |
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Employment Agreement between Volition Diagnostics and Nick Plummer, dated August 23, 2021. |
|
10-Q |
|
001-36833 |
|
10.3 |
|
11/10/21 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Power of Attorney (included on the signature page of this Report). |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
X |
||
| 77 |
| Table of Contents |
|
|
|
|
Incorporated by Reference |
|
|
||||||
|
Exhibit Number |
|
Exhibit Description |
|
Form |
|
File No. |
|
Exhibit |
|
Filing Date |
|
Filed Herewith |
|
|
|
|
|
|
|
|
|
|
|
|
|
10.1 INS |
|
XBRL Instance Document |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
101.SCH |
|
XBRL Taxonomy Extension Schema Document. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
101.CAL |
|
XBRL Taxonomy Extension Calculation Linkbase Document. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
101.DEF |
|
XBRL Taxonomy Extension Definition Linkbase Document. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
101.LAB |
|
XBRL Taxonomy Extension Label Linkbase Document. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
101.PRE |
|
XBRL Taxonomy Extension Presentation Linkbase Document. |
|
|
|
|
|
|
|
|
|
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
104 |
|
Cover Page Interactive Data File (formatted as Inline XBRL and contained in Exhibit 101) |
|
|
|
|
|
|
|
|
|
X |
# |
Indicates a management contract or compensatory plan or arrangement. |
† |
Portions of this exhibit are redacted pursuant to Item 601(a)(6) and/or Item (b)(10)(iv) under Regulation S-K. The registrant agrees to furnish supplementally any omitted schedules to the SEC upon request. |
* |
The certifications attached as Exhibit 32.1 accompany this Report pursuant to 18 U.S.C. Section 1350, as adopted pursuant to Section 906 of the Sarbanes-Oxley Act of 2002, and shall not be deemed “filed” by the registrant for purposes of Section 18 of the Exchange Act and are not to be incorporated by reference into any of the registrant’s filings under the Securities Act or the Exchange Act, irrespective of any general incorporation language contained in any such filing. |
ITEM 16. |
FORM 10-K SUMMARY |
None.
| 78 |
| Table of Contents |
SIGNATURES
Pursuant to the requirements of Section 13 or 15(d) of the Securities Exchange Act of 1934, the registrant caused this Report to be signed on its behalf by the undersigned, thereunto duly authorized.
|
VOLITIONRX LIMITED |
|
|
|
|
|
|
Dated: March 30, 2022 |
By: |
/s/ Cameron Reynolds |
|
|
|
Cameron Reynolds |
|
|
|
President, Chief Executive Officer and Director |
|
POWER OF ATTORNEY
KNOW ALL PERSONS BY THESE PRESENTS that each individual whose signature appears below constitutes and appoints Cameron Reynolds and Rodney Rootsaert, and each or either of them, acting individually, his or her true and lawful attorney-in-fact and agent, with full power of substitution, for him or her and in his or her name, place and stead, in any and all capacities, to sign any and all amendments to this Report on Form 10-K, and to file the same, with all exhibits thereto and other documents in connection therewith, with the Securities and Exchange Commission, granting unto said attorneys-in-fact and agents, and each of them, full power and authority to do and perform each and every act and thing requisite and necessary to be done in connection therewith, as fully to all intents and purposes as he or she might or could do in person, hereby ratifying and confirming all that said attorneys-in-fact and agents, or either of them, or his, her or their substitute or substitutes, may lawfully do or cause to be done or by virtue hereof.
Pursuant to the requirements of the Securities Exchange Act of 1934, this Report on Form 10-K has been signed below by the following persons in the capacities and on the date indicated.
Signature |
|
Title |
|
Date |
|
|
|
|
|
/s/ Cameron Reynolds |
|
President, Chief Executive Officer and Director |
|
March 30, 2022 |
Cameron Reynolds |
|
(Principal Executive Officer) |
|
|
|
|
|
|
|
/s/ Terig Hughes |
|
Chief Financial Officer and Treasurer |
|
March 30, 2022 |
Terig Hughes |
|
(Principal Financial and Accounting Officer) |
|
|
|
|
|
|
|
/s/ Dr. Martin Faulkes |
|
Director |
|
March 30, 2022 |
Dr. Martin Faulkes |
|
|
|
|
|
|
|
|
|
/s/ Guy Innes |
|
Director |
|
March 30, 2022 |
Guy Innes |
|
|
|
|
|
|
|
|
|
/s/ Dr. Alan Colman |
|
Director |
|
March 30, 2022 |
Dr. Alan Colman |
|
|
|
|
|
|
|
|
|
/s/ Dr. Phillip Barnes |
|
Director |
|
March 30, 2022 |
Dr. Phillip Barnes |
|
|
|
|
|
|
|
|
|
/s/ Dr. Edward Futcher |
|
Director |
|
March 30, 2022 |
Dr. Edward Futcher |
|
|
|
|
|
|
|
|
|
/s/ Kim Nguyen |
|
Director |
|
March 30, 2022 |
Kim Nguyen |
|
|
|
|
|
|
|
|
|
/s/ Richard Brudnick |
|
Director |
|
March 30, 2022 |
Richard Brudnick |
|
|
|
|
79 |