VolitionRx Limited to Present COVID-19 Data at MEDICA 2020
AUSTIN, Nov. 19, 2020 /PRNewswire/ -- VolitionRx Limited (NYSE AMERICAN: VNRX) ("Volition"), a multi-national epigenetics company developing simple, easy to use, cost effective blood tests to help diagnose a range of cancers and other diseases, has announced that Dr. Mark Eccleston, a founding scientist and Business Development Director at Volition, will today present preliminary results from its proof of concept clinical studies focused on monitoring disease progression of COVID-19 as part of the "Innovative COVID-19 Diagnostics" session at the virtual MEDICA LABMED FORUM.
Volition's initial studies demonstrated that symptomatic COVID-19 patients have elevated circulating nucleosomes and, as a result, Volition has filed a novel patent for monitoring disease progression of COVID-19 and other NETosis associated infections. Preliminary results from ongoing longitudinal COVID-19 studies are expected to be released before the end of 2020. The Company plans to utilise the results of these current trials and other ongoing studies to further its aim of developing a clinically useful product to help in the COVID-19 pandemic, and potentially in other infections with dangerous complications caused by NETosis including influenza and sepsis.
The presentation, "Circulating nucleosomes as potential prognostic markers for COVID-19 disease severity" will outline the data compiled so far to support the Company's use of its Nu.Q™ Nucleosomics™ technology to identify cell free circulating nucleosomes associated with elevated NETosis - the body's immune response to an infection - in "at risk" individuals with COVID-19.
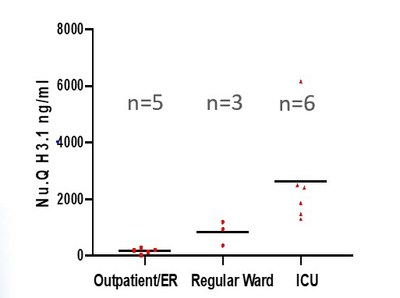
Data from two independent cohorts of COVID-19 positive patients with quantitative nucleosome immunoassays showed that nucleosomes were highly elevated in the plasma of patients with severe COVID-19, relative to healthy control subjects. The data showed that the levels of both histone 3.1 variant and citrullinated nucleosomes increased with disease severity.
The highest levels of nucleosomes were found in patients requiring artificial ventilation or extracorporeal oxygenation. Volition believes that it is possible, therefore, that nucleosomes could be used to monitor disease progression in COVID-19 positive patients and potentially other diseases such as influenza and sepsis.
Nu.Q H3.1 increased with disease severity.
Early identification and triaging of at-risk COVID-19 patients to determine those most likely to deteriorate and require critical care would enable both improved outcomes for patients and a more efficient use of critical care resources for healthcare providers.
Dr. Eccleston said: "We are very pleased to be invited to present data at the MEDICA LABMED FORUM on the work we are doing to use circulating nucleosomes to monitor COVID-19. As we see the number of cases rise again worldwide, we believe more than ever that the ability to understand disease progression and to manage resources accordingly is an important and still unmet need. We look forward to announcing further data soon."
You can view the presentation here: https://volition.com/resources/downloads/Volition-Medica-COVID-presentation.pdf
About Volition
Volition is a multi-national epigenetics company developing simple, easy to use, cost effective blood tests to help diagnose a range of cancers and other diseases. Early diagnosis has the potential to not only prolong the life of patients, but also to improve their quality of life. The tests are based on the science of NucleosomicsTM, which is the practice of identifying and measuring nucleosomes in the bloodstream or other bodily fluid - an indication that disease is present. Volition is primarily focused on human diagnostics but also has a subsidiary focused on animal diagnostics.
Volition's research and development activities are centered in Belgium, with a small laboratory in California and additional offices in Texas, London and Singapore, as the company focuses on bringing its diagnostic products to market.
For more information about Volition, visit Volition's website volition.com or connect with us via:
Twitter: https://twitter.com/volitionrx
LinkedIn: https://www.linkedin.com/company/volitionrx
Facebook: https://www.facebook.com/VolitionRx/
YouTube: https://www.youtube.com/user/VolitionRx
The contents found at Volition's website address, Twitter, LinkedIn, Facebook, and YouTube are not incorporated by reference into this document and should not be considered part of this document. The addresses for Volition's website, Twitter, LinkedIn, Facebook, and YouTube are included in this document as inactive textual references only.
Media / Investor Contacts
|
Louise Batchelor, Volition +44 (0)7557 774620 |
Scott Powell, Volition investorrelations@volition.com +1 (646) 650 1351 |
|
Jen Lewis, Pegasus +44 (0)7809 867943 |
Joseph Green, Edison Advisors +1 (646) 653 7030 |
Safe Harbor Statement
Statements in this press release may be "forward-looking statements" within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that concern matters that involve risks and uncertainties that could cause actual results to differ materially from those anticipated or projected in the forward-looking statements. Words such as "expects," "anticipates," "intends," "plans," "aims," "targets," "believes," "seeks," "estimates," "optimizing," "potential," "goal," "suggests," "could," "would," "should," "may," "will" and similar expressions identify forward-looking statements. These forward-looking statements relate to the timing, completion and delivery of data from clinical studies, the effectiveness of Volition's blood-based diagnostic and prognostic tests, and Volition's ability to develop and successfully commercialize such test platforms for early detection of cancer and other diseases as well as serving as a diagnostic or prognostic tool for COVID-19. Volition's actual results may differ materially from those indicated in these forward-looking statements due to numerous risks and uncertainties, including, without limitation, results of studies testing the efficacy of its tests. For instance, if Volition fails to develop and commercialize diagnostic or prognostic products, it may be unable to execute its plan of operations. Other risks and uncertainties include Volition's failure to obtain necessary regulatory clearances or approvals to distribute and market future products; a failure by the marketplace to accept the products in Volition's development pipeline or any other diagnostic or prognostic products Volition might develop; Volition's failure to secure adequate intellectual property protection; Volition will face fierce competition and Volition's intended products may become obsolete due to the highly competitive nature of the diagnostics market and its rapid technological change; downturns in domestic and foreign economies; and other risks identified in Volition's most recent Annual Report on Form 10-K and Quarterly Reports on Form 10-Q, as well as other documents that Volition files with the Securities and Exchange Commission. These statements are based on current expectations, estimates and projections about Volition's business based, in part, on assumptions made by management. These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict. Forward-looking statements are made as of the date of this release, and, except as required by law, Volition does not undertake an obligation to update its forward-looking statements to reflect future events or circumstances.
Nucleosomics™ and Nu.Q™ and their respective logos are trademarks and/or service marks of VolitionRx Limited and its subsidiaries. All other trademarks, service marks and trade names referred to in this press release are the property of their respective owners.
![]() View original content to download multimedia:http://www.prnewswire.com/news-releases/volitionrx-limited-to-present-covid-19-data-at-medica-2020-301177004.html
View original content to download multimedia:http://www.prnewswire.com/news-releases/volitionrx-limited-to-present-covid-19-data-at-medica-2020-301177004.html
SOURCE VolitionRx Ltd
Released November 19, 2020